Isolation and Characterization of Two Bacteriophages and Their Preventive Effects against Pathogenic Vibrio coralliilyticus Causing Mortality of Pacific Oyster (Crassostrea gigas) Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Phage Isolation, Purification, and Propagation
2.3. Phage Characterization
2.3.1. Host Range and Efficiency of Plating (EOP)
2.3.2. Electron Microscopy
2.3.3. Phage Stability Test
2.3.4. Host Cell Lysis Test
2.4. Prophylactic Efficacy of the Isolated Phages
2.5. Statistical Analysis
3. Results
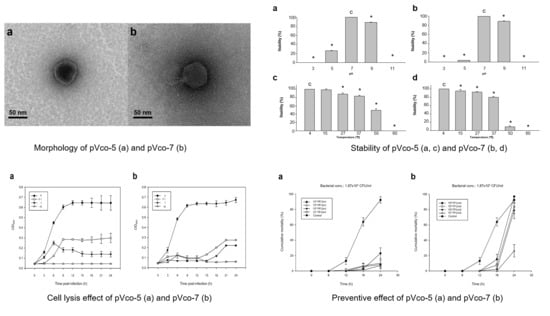
3.1. Phage Isolation and Morphology
3.2. Characterization of pVco-5 and pVco-7
3.2.1. Host Range and EOPs
3.2.2. Stability Test
3.2.3. Bacterial Cell Lysis Test
3.3. Preventive Efficacy of Pre-phage Treatment
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- FAO Fish Stat. Global Aquaculture Production for Species (Tonnes): Pacific Oyster. 2019. Available online: http://www.fao.org/fishery/topic/16140/en (accessed on 31 October 2019).
- Jee, B.Y.; Lee, S.J.; Cho, M.Y.; Lee, S.J.; Kim, J.W.; Choi, S.H.; Kim, K.H. Detection of Ostreid Herpesvirus 1 from adult Pacific oysters Crassostrea gigas cultured in Korea. Fish. Aquat. Sci. 2013, 16, 131–135. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.; Park, J.; Yu, H.; Hur, Y.; Arzul, I.; Couraleau, Y.; Park, M. Ostreid herpesvirus 1 infection in farmed Pacific oyster larvae Crassostrea gigas (Thunberg) in Korea. J. Fish Dis. 2013, 36, 969–972. [Google Scholar]
- Kim, H.J.; Jun, J.W.; Giri, S.S.; Yun, S.; Kim, S.G.; Kim, S.W.; Kang, J.W.; Han, S.J.; Kwon, J.; Oh, W.T.; et al. Mass mortality in Korean bay scallop (Argopecten irradians) associated with Ostreid Herpesvirus-1 uVar. Transbound. Emerg. Dis. 2019, 66, 1442–1448. [Google Scholar]
- Kim, H.J.; Jun, J.W.; Giri, S.S.; Chi, C.; Yun, S.; Kim, S.G.; Kim, S.W.; Han, S.J.; Kwon, J.; Oh, W.T.; et al. Identification and genome analysis of Vibrio coralliilyticus causing mortality of Pacific oyster (Crassostrea gigas) larvae. Pathogens 2020, 9, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elston, R.A.; Hasegawa, H.; Humphrey, K.L.; Polyak, I.K.; Häse, C.C. Re-emergence of Vibrio tubiashii in bivalve shellfish aquaculture: Severity, environmental drivers, geographic extent and management. Dis. Aquat. Org. 2008, 82, 119–134. [Google Scholar] [CrossRef]
- Richards, G.P.; Watson, M.A.; Needleman, D.S.; Church, K.M.; Häse, C.C. Mortalities of Eastern and Pacific oyster larvae caused by the pathogens Vibrio coralliilyticus and Vibrio tubiashii. Appl. Environ. Microbiol. 2015, 81, 292–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugumar, G.; Nakai, T.; Hirata, Y.; Matsubara, D.; Muroga, K. Vibrio splendidus biovar II as the causative agent of bacillary necrosis of Japanese oyster Crassostrea gigas larvae. Dis. Aquat. Org. 1998, 33, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Travers, M.-A.; Achour, R.M.; Haffner, P.; Tourbiez, D.; Cassone, A.-L.; Morga, B.; Fruitier-Arnaudin, I. First description of French V. tubiashii strains pathogenic to mollusk: I. Characterization of isolates and detection during mortality events. J. Invertebr. Pathol. 2014, 123, 38–48. [Google Scholar] [CrossRef] [Green Version]
- Tubiash, H.S.; Chanley, P.E.; Leifson, E. Bacillary necrosis, a disease of larval and juvenile bivalve mollusks I. Etiology and epizootiology. J. Bacteriol. 1965, 90, 1036–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubert, J.; Osorio, C.R.; Prado, S.; Barja, J.L. Persistence of antibiotic resistant Vibrio spp. in shellfish hatchery environment. Microb. Ecol. 2016, 72, 851–860. [Google Scholar] [CrossRef]
- Matsubara, D.; Tanaka, M.; Soumyou, Y.; Hirakawa, K.; Doi, R.; Nakai, T. Therapeutic effects of antimicrobial compounds against bacillary necrosis of larval Pacific oyster. Fish. Pathol. 2002, 37, 183–188. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.H.; Jun, J.W.; Giri, S.S.; Chi, C.; Yun, S.; Kim, S.G.; Kim, S.H.; Kang, J.W.; Jeong, D.G.; et al. Complete genome sequence of Vibrio coralliilyticus 58, Isolated from Pacific oyster (Crassostrea gigas) larvae. Genome Announc. 2017, 5, e00437-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Defoirdt, T.; Sorgeloos, P.; Bossier, P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr. Opin. Microbiol. 2011, 14, 251–258. [Google Scholar] [CrossRef]
- Giraud, E.; Blanc, G.; Bouju-Albert, A.; Weill, F.X.; Donnay-Moreno, C. Mechanisms of quinolone resistance and clonal relationship among Aeromonas salmonicida strains isolated from reared fish with furunculosis. J. Med. Microbiol. 2004, 53, 895–901. [Google Scholar] [CrossRef] [Green Version]
- Cohen, Y.; Joseph Pollock, F.; Rosenberg, E.; Bourne, D.G. Phage therapy treatment of the coral pathogen Vibrio coralliilyticus. Microbiologyopen 2013, 2, 64–74. [Google Scholar] [CrossRef]
- Kim, H.J.; Jun, J.W.; Giri, S.S.; Chi, C.; Yun, S.; Kim, S.G.; Kim, S.W.; Kang, J.W.; Han, S.J.; Kwon, J.; et al. Application of the bacteriophage pVco-14 to prevent Vibrio coralliilyticus infection in Pacific oyster (Crassostrea gigas) larvae. J. Invertebr. Pathol. 2019, 167, 107244. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, X.; Zhang, J.; Wang, X.; Wang, L.; Cao, Z.; Xu, Y. Use of phages to control Vibrio splendidus infection in the juvenile sea cucumber Apostichopus japonicus. Fish. Shellfish Immunol. 2016, 54, 302–311. [Google Scholar] [CrossRef]
- Patil, J.R.; Desai, S.N.; Roy, P.; Durgaiah, M.; Saravanan, R.S.; Vipra, A. Simulated hatchery system to assess bacteriophage efficacy against Vibrio harveyi. Dis. Aquat. Org. 2014, 112, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Jacquemot, L.; Bettarel, Y.; Monjol, J.; Corre, E.; Halary, S.; Desnues, C.; Bouvier, T.; Ferrier-Pages, C.; Baudoux, A.-C. Therapeutic potential of a new jumbo phage that infects Vibrio coralliilyticus, a widespread coral pathogen. Front. Microbiol. 2018, 9, 2501. [Google Scholar] [CrossRef] [Green Version]
- Jun, J.W.; Han, J.E.; Giri, S.S.; Tang, K.F.; Zhou, X.; Aranguren, L.F.; Kim, H.J.; Yun, S.; Chi, C.; Kim, S.G.; et al. Phage application for the protection from Acute Hepatopancreatic Necrosis Disease (AHPND) in Penaeus vannamei. Indian J. Microbiol. 2018, 58, 114–117. [Google Scholar] [CrossRef]
- Kesarcodi-Watson, A.; Miner, P.; Nicolas, J.-L.; Robert, R. Protective effect of four potential probiotics against pathogen-challenge of the larvae of three bivalves: Pacific oyster (Crassostrea gigas), flat oyster (Ostrea edulis) and scallop (Pecten maximus). Aquaculture 2012, 344, 29–34. [Google Scholar] [CrossRef]
- Takahashi, K.G.; Nakamura, A.; Mori, K. Inhibitory Effects of Ovoglobulins on Bacillary Necrosis in Larvae of the Pacific Oyster, Crassostrea gigas. J. Invertebr. Pathol. 2000, 75, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Chhibber, S.; Kaur, S.; Kumari, S. Therapeutic potential of bacteriophage in treating Klebsiella pneumoniae B5055-mediated lobar pneumonia in mice. J. Med. Microbiol. 2008, 57, 1508–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, S.; Harjai, K.; Chhibber, S. Efficacy of bacteriophage treatment in murine burn wound infection induced by Klebsiella pneumoniae. J. Microbiol. Biotechnol. 2009, 19, 622–628. [Google Scholar]
- Kutter, E.; Sulakvelidze, A. Bacteriophages: Biology and Applications; CRC Press: New York, NY, USA, 2004. [Google Scholar]
- Hoai, T.D.; Mitomi, K.; Nishiki, I.; Yoshida, T. A lytic bacteriophage of the newly emerging rainbow trout pathogen Weissella ceti. Virus Res. 2018, 247, 34–39. [Google Scholar] [CrossRef]
- Jun, J.W.; Kim, J.H.; Shin, S.P.; Han, J.E.; Chai, J.Y.; Park, S.C. Protective effects of the Aeromonas phages pAh1-C and pAh6-C against mass mortality of the cyprinid loach (Misgurnus anguillicaudatus) caused by Aeromonas hydrophila. Aquaculture 2013, 416, 289–295. [Google Scholar] [CrossRef]
- Park, S.C.; Nakai, T. Bacteriophage control of Pseudomonas plecoglossicida infection in ayu Plecoglossus altivelis. Dis. Aquat. Org. 2003, 53, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Park, S.C.; Shimamura, I.; Fukunaga, M.; Mori, K.I.; Nakai, T. Isolation of bacteriophages specific to a fish pathogen, Pseudomonas plecoglossicida, as a candidate for disease control. Appl. Environ. Microbiol. 2000, 66, 1416–1422. [Google Scholar] [CrossRef] [Green Version]
- Jun, J.W.; Shin, T.H.; Kim, J.H.; Shin, S.P.; Han, J.E.; Heo, G.J.; De Zoysa, M.; Shin, G.W.; Chai, J.Y.; Park, S.C. Bacteriophage therapy of a Vibrio parahaemolyticus infection caused by a multiple-antibiotic-resistant O3:K6 pandemic clinical strain. J. Infect. Dis. 2014, 210, 72–78. [Google Scholar] [CrossRef]
- Takaoka, O.; Ji, S.-C.; Ishimaru, K.; Lee, S.-W.; Jeong, G.-S.; Ito, J.; Biswas, A.; Takii, K. Effect of rotifer enrichment with herbal extracts on growth and resistance of red sea bream, Pagrus major (Temminck Schlegel) larvae against Vibrio anguillarum. Aquac. Res. 2011, 42, 1824–1829. [Google Scholar] [CrossRef]
- Nakajima, K.; Muroga, K.; Hancock, R.E.W. Comparison of fatty acid, protein, and serological properties distinguishing outer membranes of Pseudomonas anguilliseptica strains from those of fish pathogens and other Pseudomnonads. Int. J. Syst. Bacteriol. 1983, 33, 1–8. [Google Scholar] [CrossRef]
- Austin, B.; Austin, D.A.; Blanch, A.R.; Cerda, M.; Grimont, F.; Grimont, P.A.D.; Jofre, J.; Koblavy, S.; Larsen, J.L.; Pedersen, K.; et al. A comparison of methods for the typing of fish-pathogenic Vibrio spp. Syst. Appl. Microbiol. 1997, 20, 89–101. [Google Scholar] [CrossRef]
- Song, Y.L.; Lee, S.P.; Lin, Y.T.; Chen, C.C. Enzyme immunoassay for shrimp vibriosis. Dis. Aquat. Org. 1992, 14, 43–50. [Google Scholar] [CrossRef]
- Cerveny, K.E.; DePaola, A.; Duckworth, D.H.; Gulig, P.A. Phage therapy of local and systemic disease caused by Vibrio vulnificus in iron-dextran-treated mice. Infect. Immun. 2002, 70, 6251–6262. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.H. Bacteriophages; Interscience Publisher: New York, NY, USA, 1959. [Google Scholar]
- Sambrook, J.; Russell, D.W.; Russell, D.W. Molecular cloning: A laboratory manual (3-volume set). Immunology 2001, 49, 895–909. [Google Scholar]
- Verma, V.; Harjai, K.; Chhibber, S. Characterization of a T7-like lytic bacteriophage of Klebsiella pneumoniae B5055: A potential therapeutic agent. Curr. Microbiol. 2009, 59, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, H.W. 5500 Phages examined in the electron microscope. Arch. Virol. 2007, 152, 227–243. [Google Scholar] [CrossRef]
- Arzul, I.; Renault, T.; Lipart, C. Experimental herpes-like viral infections in marine bivalves: Demonstration of interspecies transmission. Dis. Aquat. Org. 2001, 46, 1–6. [Google Scholar] [CrossRef] [PubMed]




| Bacterial Species | Strain | Host Range a (EOPs b) | Source a | |
|---|---|---|---|---|
| pVco-5 | pVco-7 | |||
| V. coralliilyticus | 58 | +(1) | +(1) | [8] |
| V. coralliilyticus | 59 | +(0.94 ± 0.75) | +(1 ± 0.12) | [8] |
| V. coralliilyticus | 60 | +(1.03 ± 0.13) | +(0.91 ± 0.09) | [8] |
| V. coralliilyticus | Q1 | +(0.9 ± 0.44) | +(0.53 ± 0.12) | [8] |
| V. tubiashii | ATCC19109 | - | - | ATCC |
| V. parahaemolyticus | CRS09-17 | - | - | [31] |
| V. alginolyticius | rM8402 | - | - | [32] |
| V. anguillarum | HT7602 | - | - | [33] |
| V. cholerae | PS-7701 | - | - | [34] |
| V. harveyi | ATCC14126 | - | - | ATCC |
| V. vulnificus | ET7618 | - | [35] | |
| Echerichia coli | ATCC25922 | - | - | ATCC |
| Phage | Phage conc. (PFU/mL) | Vco 58 conc. (CFU/mL) | Mortality (24 h Post-Infection) (%, Mean ± SD) |
|---|---|---|---|
| pVco-5 | 1.25 × 104 | 1.87 × 105 | 23.00 ± 7.20 |
| 1.25 × 105 | 1.87 × 105 | 7.18 ± 1.28 | |
| 1.25 × 106 | 1.87 × 105 | 8.25 ± 5.55 | |
| 1.25 × 107 | 1.87 × 105 | 10.09 ± 5.71 | |
| pVco-7 | 1.44 × 104 | 1.87 × 105 | 97.20 ± 1.21 |
| 1.44 × 105 | 1.87 × 105 | 83.80 ± 8.90 | |
| 1.44 × 106 | 1.87 × 105 | 78.85 ± 10.67 | |
| 1.44 × 107 | 1.87 × 105 | 26.32 ± 8.14 | |
| Control | 0 | 1.87 × 105 | 92.49 ± 4.12 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.J.; Giri, S.S.; Kim, S.G.; Kim, S.W.; Kwon, J.; Lee, S.B.; Park, S.C. Isolation and Characterization of Two Bacteriophages and Their Preventive Effects against Pathogenic Vibrio coralliilyticus Causing Mortality of Pacific Oyster (Crassostrea gigas) Larvae. Microorganisms 2020, 8, 926. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8060926
Kim HJ, Giri SS, Kim SG, Kim SW, Kwon J, Lee SB, Park SC. Isolation and Characterization of Two Bacteriophages and Their Preventive Effects against Pathogenic Vibrio coralliilyticus Causing Mortality of Pacific Oyster (Crassostrea gigas) Larvae. Microorganisms. 2020; 8(6):926. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8060926
Chicago/Turabian StyleKim, Hyoun Joong, Sib Sankar Giri, Sang Guen Kim, Sang Wha Kim, Jun Kwon, Sung Bin Lee, and Se Chang Park. 2020. "Isolation and Characterization of Two Bacteriophages and Their Preventive Effects against Pathogenic Vibrio coralliilyticus Causing Mortality of Pacific Oyster (Crassostrea gigas) Larvae" Microorganisms 8, no. 6: 926. https://0-doi-org.brum.beds.ac.uk/10.3390/microorganisms8060926