In Vivo and In Vitro Antioxidant Activities of Methanol Extracts from Olive Leaves on Caenorhabditis elegans
Abstract
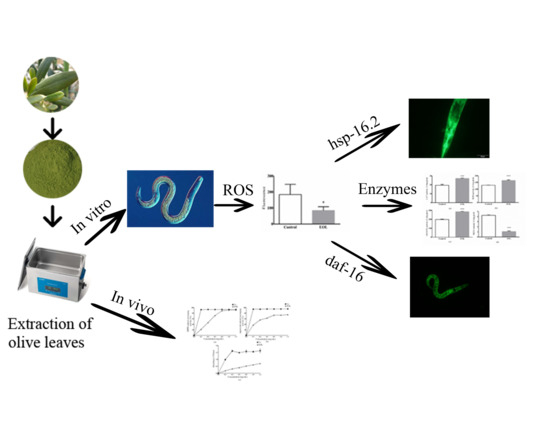
:1. Introduction
2. Results and Discussion
2.1. Components of EOL
2.2. In Vitro Antioxidant Activities
2.3. In Vivo Biological Activities
2.3.1. Determination of a Suitable Concentration
2.3.2. Verification of the Non-Toxic Concentration
2.3.3. Survival under Thermal Stress
2.3.4. The ROS Level
2.3.5. The Expression of HSP-16.2
2.3.6. The Antioxidant Enzymes Activities and MDA Levels
2.3.7. Nuclear Translocation of daf-16::GFP
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Methanol Extraction from Olive Leaves
3.3. Chemical Class Determination of EOL
3.3.1. Polyphenols Content
3.3.2. Flavonoids Content
3.3.3. Soluble Proteins Content
3.3.4. Soluble Sugars Content
3.3.5. Free Amino Acids Content
3.4. In Vitro Antioxidant Activity Assays
3.4.1. DPPH Radical Scavenging Assay
3.4.2. Superoxide Radical Scavenging Assay
3.4.3. Reducing Power Assay
3.5. In Vivo Antioxidant Activity Assays
3.5.1. C. elegans and Culture Conditions
3.5.2. Food Clearance Assay
3.5.3. Fertility Assay
3.5.4. Thermal Stress Assay
3.5.5. Determination of the ROS Level
3.5.6. Visualization of the HSP-16.2::GFP
3.5.7. Measurement of Antioxidant Enzymes and the MDA Level
3.5.8. Nuclear Localization of daf-16
3.6. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ying, J.; Liang, G. Nutritive composition and health care function of olive oil. Acad. Period. Farm Prod. Prcess. 2012, 6, 94–96. [Google Scholar] [CrossRef]
- Papadopoulos, G.; Boskou, D. Antioxidant effect of natural phenols on olive oil. J. Am. Oil Chem. Soc. 1991, 68, 669–671. [Google Scholar] [CrossRef]
- Hull, W.E. Olive-oil consumption and health: The possible role of antioxidants. Lancet Oncol. 2000, 1, 107–112. [Google Scholar] [CrossRef]
- Owen, R.W.; Giacosa, A.; Hull, W.E.; Haubner, R.; Spiegelhalder, B.; Bartsch, H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. Eur. J. Cancer 2000, 36, 1235–1247. [Google Scholar] [CrossRef]
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef]
- María, D.P.; Feliciano, P.C.; María, D.L.C. Selective ultrasound-enhanced enzymatic hydrolysis of oleuropein to its aglycon in olive (Olea europaea L.) leaf extracts. Food Chem. 2017, 220, 282–288. [Google Scholar] [CrossRef]
- Caixia, G.; Chengzhang, W.; Chengying, J. Chemical composition and processing utilization of olive. For. Sci. Technol. Dev. 2006, 20, 1–4. [Google Scholar] [CrossRef]
- Jianzhong, Y.; Chengzhang, W.; Hongxia, C.; Hao, Z. Variation Rule of Hydroxytyrosol Content in Olive Leaves. Chem. Ind. For. Prod. 2011, 31, 69–74. [Google Scholar] [CrossRef]
- Rao, F.; Yuting, Z.; Yiran, G.; Fengxai, L.; Fang, C. Determination of phenolic contents and antioxidant activities of extracts of Jatropha curcas L. seed shell, a by-product, a new source of natural antioxidant. Ind. Crop. Prod. 2014, 58, 265–270. [Google Scholar] [CrossRef]
- Aruoma, O.I. Free radicals, oxidative stress, and antioxidants in human health and disease. J. Am. Oil Chem. Soc. 1998, 75, 199–212. [Google Scholar] [CrossRef]
- Maia, T.; Phillip, F.P.; Debebe, G.; Jayashree, N.; David, R.H. Reactive oxygen species cerebral autoregulation in health and disease. Pediatr. Clin. N. Am. 2006, 53, 1029–1037. [Google Scholar] [CrossRef]
- Harman, D. The Biologic Clock: The Mitochondria? J. Am. Geriatr. Soc. 1972, 20, 145–147. [Google Scholar] [CrossRef]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Mark, T.D.; Milan, M.; Joshua, T. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell B 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef]
- Simona, D.M.; Carmen, F.; Franco, Z.; Antonella, N.; Lina, A.; Gennaro, R.; Maria, I. Antioxidant activity and chemical components as potential anticancer agents in the olive leaf (Olea europaea L. cv Leccino.) Decoction. Anti-Cancer Agent Med. Chem. 2014, 14, 1376–1385. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Zheng, J.; Park, Y. Caenorhabditis elegans: A convenient in vivo model for assessing the impact of food bioactive components on obesity, aging, and alzheimer’s disease. Annu. Rev. Food Sci. Technol. 2017, 9, 1–22. [Google Scholar] [CrossRef]
- Lithgow, G.J.; Walker, G.A. Stress resistance as a determinate of C. elegans lifespan. Mech. Ageing Dev. 2002, 123, 765–771. [Google Scholar] [CrossRef]
- Liao, H.C.; Yu, C.W.; Chu, Y.J.; Li, W.H.; Hsieh, Y.C.; Wang, T.T. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech. Ageing Dev. 2011, 132, 480–487. [Google Scholar] [CrossRef]
- Yanhong, L.; Bo, J.; Tao, Z.; Wanmeng, M.; Jian, L. Antioxidant and free radical-scavenging activities of chickpea protein hydrolysate (CPH). Food Chem. 2007, 106, 444–450. [Google Scholar] [CrossRef]
- Zhang, Y.; Mi, D.-Y.; Wang, J.; Luo, Y.-P.; Yang, X.; Dong, S.; Ma, X.-M.; Dong, Z. Constituent and effects of polysaccharides isolated from Sophora moorcroftiana, seeds on lifespan, reproduction, stress resistance, and antimicrobial capacity in Caenorhabditis elegans. Chin. J. Nat. Med. 2018, 16, 252–260. [Google Scholar] [CrossRef]
- Ishikado, A.; Sono, Y.; Matsumoto, M.; Stacey, R.S.; Aya, O.; Masashi, G.; George, L.K.; Blackwell, T.K.; Taketoshi, M. Willow bark extract increases antioxidant enzymes and reduces oxidative stress through activation of Nrf2 in vascular endothelial cells and Caenorhabditis elegans. Free Radical Biol. Med. 2013, 65, 1506–1515. [Google Scholar] [CrossRef]
- Cañuelo, A.; López, B.G.; Liñán, P.P.; Lara, E.M.; Siles, E.; Vizuete, A.M. Tyrosol, a main phenol present in extra virgin olive oil, increases lifespan and stress resistance in Caenorhabditis elegans. Mech. Ageing Dev. 2012, 133, 563–574. [Google Scholar] [CrossRef]
- Le, F.F.; Tena, M.T.; Ríos, A.; Valcarcel, M. Supercritical fluid extraction of phenol compounds from olive leaves. Talanta 1998, 46, 1123–1130. [Google Scholar] [CrossRef]
- Prabhakar, K.R.; Veeresh, V.P.; Vipan, K.; Sudheer, M.; Priyadarsini, K.I.; Satish, R.B.S.S.; Unnikrishnan, M.K. Bioactivity-guided fractionation of Coronopus didymus: A free radical scavenging perspective. Phytomedicine 2006, 13, 591–595. [Google Scholar] [CrossRef]
- González, C.S. Methods used to evaluate the free radical sacavening activity in foods and biological systems: Review. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Brahmi, F.; Mechri, B.; Dabbou, S.; Dhibi, M.; Hammami, M. The efficacy of phenolics compounds with different polarities as antioxidants from olive leaves depending on seasonal variations. Ind. Crop. Prod. 2012, 38, 146–152. [Google Scholar] [CrossRef]
- Abaza, L.; Youssef, B.N.; Djebali, H.M.; Faouzia, H.; Methenmi, K. Chétoui olive leaf extracts: Influence of the solvent type on phenolics and antioxidant activities. Grasas Y Aceites 2011, 62, 96–104. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Klaus, B. Antioxidant Properties of Various Solvent Extracts of Total Phenolic Constituents from Three Different Agroclimatic Origins of Drumstick Tree (Moringa oleifera Lam.) Leaves. J. Agric. Food Chem. 2003, 51, 2144. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D.; Tsai, C.L. Relationship between antioxidant activity and maturity of peanut hulls. J. Agric. Food Chem. 1993, 41, 67–70. [Google Scholar] [CrossRef]
- Falleh, H.; Ksouri, R.; Chaieb, K.; Bouraoui, N.K.; Trabelsi, N.; Boulaaba, M.; Abdelly, C. Phenolic composition of Cynara cardunculus, L. organs, and their biological activities. CR Biol. 2008, 331, 372–379. [Google Scholar] [CrossRef]
- Xian, X.; Cao, J.; Zheng, Y.; Quanxi, W.; Jianbo, X. Flavonoid concentrations and bioactivity of flavonoid extracts from 19 species of ferns from China. Ind. Crop. Prod. 2014, 58, 91–98. [Google Scholar] [CrossRef]
- Nadia, D.; Nicola, M.; Daniela, R.; Immacolata, F.; Nunziatina, D.T.; Souad, A.; Lorella, S.; Luigi, M. Phenolic compounds from Olea europaea L. possess antioxidant activity and inhibit carbohydrate metabolizing enzymes in vitro. Evid. Based Complement Alternat. Med. 2015, 2015, 684925. [Google Scholar] [CrossRef]
- Vayndorf, E.M.; Lee, S.S.; Liu, R.H. Whole apple extracts increase lifespan, healthspan and resistance to stress in Caenorhabditis elegans. J. Funct. Foods 2013, 5, 1235–1243. [Google Scholar] [CrossRef]
- Jara, P.M.; González, M.S.; Escudero, G.M.; Hernanz, D.; Dueñas, M.; González, P.A.; Heredia, F.; Santos, B.C. Study of Zalema Grape Pomace: Phenolic Composition and Biological Effects in Caenorhabditis elegans. J. Agric. Food Chem. 2013, 61, 5114–5121. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Thirumurugan, K. Longevity promoting efficacies of different plant extracts in lower model organisms. Mech. Ageing Dev. 2018, 171, 47–57. [Google Scholar] [CrossRef]
- Shiling, F.; Haoran, C.; Zhou, X.; Shian, S.; Ming, Y.; Jing, L.; Chunbang, D. Thermal stress resistance and aging effects of Panax notoginseng, polysaccharides on Caenorhabditis elegans. Int. J. Biol. Macromol. 2015, 81, 188–194. [Google Scholar] [CrossRef]
- Shiling, F.; Haoran, C.; Zhou, X.; Ming, Y.; Yan, H.; Jinqiu, L.; Ruiwu, Y.; Lijun, Z.; Chunbang, D. Panax notoginseng, polysaccharide increases stress resistance and extends lifespan in Caenorhabditis elegans. J. Funct. Foods 2018, 45, 15–23. [Google Scholar] [CrossRef]
- Wulf, D. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [Green Version]
- Hsu, A.L. Regulation of Aging and Age-Related Disease by DAF-16 and Heat-Shock Factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef]
- Patrícia, F.B.; Giovanna, M.M.S.; Franciny, A.P.; Rodrigo, M.C.; Cecília, V.N.; Riva, P.O. Guarana (Paullinia cupana) Extract Protects Caenorhabditis elegans Models for Alzheimer Disease and Huntington Disease through Activation of Antioxidant and Protein Degradation Pathways. Oxid. Med. Cell. Longev. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Naoaki, I.; Nanami, S.M.; Kohichiro, M.; Kayo, Y.; Takamasa, I.; Philip, S.H.; Satoru, F. Coenzyme Q10 can prolong C. elegans lifespan by lowering oxidative stress. Mech. Ageing Dev. 2004, 125, 41–46. [Google Scholar] [CrossRef]
- Zhuanhua, W.; Xiaoli, M.; Jiao, L.; Xiaodong, C. Peptides from sesame cake extend healthspan of Caenorhabditis elegans, via upregulation of skn-1, and inhibition of intracellular ROS levels. Exp. Gerontol. 2016, 82, 139–149. [Google Scholar] [CrossRef]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef]
- Celino, F.T.; Yamaguchi, S.; Miura, C.; Ohta, T.; Tozawa, Y.; Iwai, T.; Miura, T. Tolerance of spermatogonia to oxidative stress is due to high levels of Zn and Cu/Zn superoxide dismutase. PLoS ONE 2012, 6, e16938. [Google Scholar] [CrossRef]
- Xia, X.F.; Zheng, J.J.; Shao, G.M.; Wang, J.L.; Liu, X.S.; Wang, Y.F. Cloning and functional analysis of glutathione peroxidase gene in red swamp crayfish Procambarus clarkii. Fish Shellfish Immunol. 2013, 34, 1587–1595. [Google Scholar] [CrossRef]
- Bokov, A.; Chaudhuri, A.; Richardson, A. The role of oxidative damage and stress in aging. Mech. Ageing Dev. 2004, 125, 811–826. [Google Scholar] [CrossRef]
- Ogg, S.; Paradis, S.; Gottlieb, S.; Patterson, G.I.; Lee, L.; Tissenbaum, H.A.; Ruvkun, G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 1997, 389, 994–999. [Google Scholar] [CrossRef]
- Wei, C.; Hongru, L.; Congmin, W.; Xiaohua, L.; Menglu, S.; Zhenzhou, Y.; Xinyan, C.; Hongbing, W. Echinacoside, a phenylethanoid glycoside from Cistanche deserticola, extends lifespan of Caenorhabditis elegans and protects from Aβ-induced toxicity. Biogerontology 2017, 19, 47–65. [Google Scholar] [CrossRef]
- Wang, B.; Qu, J.; Luo, S.; Feng, S.; Li, T.; Yuan, M.; Huang, Y.; Liao, J.; Yang, R.; Ding, C. Optimization of ultrasound-assisted extraction of flavonoids from olive (Olea europaea L.) leaves, and evaluation of their antioxidant and anticancer Activities. Molecules 2018, 23, 2513. [Google Scholar] [CrossRef]
- Popova, M.; Bankova, V.; Butovska, D.; Petkov, V.; Damyanova, B.N.; Sabatini, A.G.; Marcazzan, G.L.; Bogdanov, S. Validated methods for the quantification of biologically active constituents of Poplar-type propolis. Phytochem. Anal. 2004, 15, 235–240. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Grintzalis, K.; Georgiou, C.D.; Schneider, Y.J. An accurate and sensitive Coomassie Brilliant Blue G-250-based assay for protein determination. Anal. Biochem. 2015, 480, 28–30. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Yemm, E.W.; Cocking, E.C. The determination of amino-acids with ninhydrin. Analyst 1955, 80, 209–214. [Google Scholar] [CrossRef]
- Brand, W.W.M.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Jia, Z.; Tang, M.; Wu, J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Moein, M.R.; Moein, S.; Ahmadizadeh, S. Radical scavenging and reducing power of Salvia mirzayanii subfractions. Molecules 2008, 12, 20804–20813. [Google Scholar] [CrossRef]
- Portadelariva, M.; Fontrodona, L.; Villanueva, A.; Ceron, J. Basic Caenorhabditis elegans methods: Synchronization and observation. J. Vis. Exp. 2012, 64, e4019. [Google Scholar] [CrossRef]
- Cindy, V.; Hemant, V.; Nicola, W.; Emily, A.B.; Brent, R.S.; Anne, C.H. Identification of potential therapeutic drugs for huntington’s disease using Caenorhabditis elegans. PLoS ONE 2007, 2, e504. [Google Scholar] [CrossRef]
- Bany, I.A.; Dong, M.Q.; Koelle, M.R. Genetic and cellular basis for acetylcholine inhibition of Caenorhabditis elegans egg-laying behavior. J. Neurosci. 2003, 23, 8060–8069. [Google Scholar] [CrossRef] [PubMed]
- Mekheimer, R.A.; Sayed, A.A.; Ahmed, E.A. Novel 1,2,4-triazolo[1,5-a]pyridines and their fused ring systems attenuate oxidative stress and prolong lifespan of Caenorhabiditis elegans. J. Med. Chem. 2012, 55, 4169–4177. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the EOL is available from the authors. |










| Compounds | Standard Caves | Contents |
| Polyphenols | y = 1.6166x + 0.0147 | 41.77 ± 2.38% |
| Flavonoids | y = 0.1793x − 0.0012 | 30.01 ± 0.76% |
| Soluble proteins | y = 1.0645x + 0.0296 | 20.40 ± 0.69% |
| Soluble sugars | y = 5.7114x + 0.0904 | 14.14 ± 0.29% |
| Free amino acids | y = 1.6031x − 0.1964 | 0.09 ± 0.02% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, S.; Jiang, X.; Jia, L.; Tan, C.; Li, M.; Yang, Q.; Du, Y.; Ding, C. In Vivo and In Vitro Antioxidant Activities of Methanol Extracts from Olive Leaves on Caenorhabditis elegans. Molecules 2019, 24, 704. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24040704
Luo S, Jiang X, Jia L, Tan C, Li M, Yang Q, Du Y, Ding C. In Vivo and In Vitro Antioxidant Activities of Methanol Extracts from Olive Leaves on Caenorhabditis elegans. Molecules. 2019; 24(4):704. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24040704
Chicago/Turabian StyleLuo, Siyuan, Xuelian Jiang, Liping Jia, Chengyue Tan, Min Li, Qiuyu Yang, Yanlin Du, and Chunbang Ding. 2019. "In Vivo and In Vitro Antioxidant Activities of Methanol Extracts from Olive Leaves on Caenorhabditis elegans" Molecules 24, no. 4: 704. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules24040704