Lignans of Sesame (Sesamum indicum L.): A Comprehensive Review
Abstract
:1. Introduction
2. Chemistry of Sesame Lignans
2.1. Structures and Chemical Properties of Lignans of Sesamum indicum
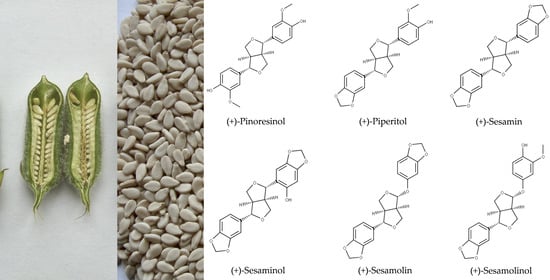
2.1.1. Aglycons
2.1.2. Lignan Glucosides
2.1.3. Does Sesame Contain Pinoresinol Monoglucoside?
2.1.4. Bias of Biomedical Research on Pinoresinol Diglucoside
2.2. Transformation and Degradation of Sesame Lignans
2.2.1. Transformation and Degradation of Lignans during Seed and Oil Processing
2.2.2. Enzymatic and Alkaline Hydrolysis of Lignan Glucosides
2.2.3. Transformation of Ligans in the Body of Mammals
2.3. Total Synthesis and Industrial Production
2.4. Stabilization of Fats by Sesame Oil and Unexpected Discovery of Sesame Lignans in Diverse Oils
2.5. Extraction, Analysis, and Purification
2.5.1. Extraction of Lignans from Seeds and Oil of Sesame
2.5.2. Color Tests and Chromatographic Methods for Lignans Analysis
2.5.3. Purification of Sesame Lignans
2.6. Variation in Lignan Content among Accessions of Sesame
2.7. Lignans in Other Tissues and Organs of Sesamum indicum
2.8. Lignans in Wild Relatives of Sesame
3. Biosynthesis of Lignans
3.1. Biosynthesis of Furofuran Lignans in Sesame
3.2. Biosynthesis of Other Lignans in Sesame
3.3. Biosynthesis of Lignans in Other Plant Species
4. Genetics of Lignan Synthesis
4.1. Genes Involved in Lignan Synthesis in Sesame
4.2. Expression of Genes of the Lignan Biosynthetic Pathway in Sesame
4.3. Molecular Markers and Heritability of Lignan Synthesis
5. Biological Activities of Lignans
5.1. Lignans as Health Promoting Agents: From Folk Medicine to Food Additives
5.2. Biological Activities of Lignans in Mammals and Their Applications
5.2.1. Antioxidative Activity
5.2.2. Estrogenic Effects, Alleviation of Postmenopausal Syndrome, and Antiestrogenic Effects
5.2.3. Sesame Lignans and Cancer
5.2.4. Neuroprotection
5.2.5. Cardioprotection and Reduction of Risk of Cardiovascular Diseases by Lowering Blood Pressure, Lipogenesis, and Cholesterol Level
5.2.6. Antiaging Effects
5.2.7. Pain Relieve, Anti inflammatory Effects and Wound Healing
5.2.8. Miscillaneous Activities: Hepatoprotection, Hypoglucemic Effect, Anti-Osteoporesis Effects, Protection of Cartilage, and Alleviation of Alcohol Sickness
5.2.9. Harmful Effects of Sesame Lignans in Humans
5.3. Antimicrobial Effects—Application and Therapeutic Potential
5.4. Biological Activites of Lignans in Insects: Synergy with Insecticides and Antifeedant Effects
6. Conceivable Biological Functions of Lignans in Sesame Plants
6.1. Resistance against Diseases
6.2. Resistance against Herbivores
6.3. Protection from Radiation Damage
6.4. Developmental Control in Seeds
7. Outlook: Biotechnology in Lignan Production
7.1. Prospects for Genetic Engineering of Sesame Plants
7.2. Cell and Hairy Root Cultures for Lignan Production
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bedigian, D.; Harlan, J.R. Evidence for cultivation of sesame in the ancient world. Econ. Bot. 1986, 40, 137–154. [Google Scholar] [CrossRef]
- Nayar, N.M.; Mehra, K.L. Sesame: Its uses, botany, cytogenetics and origin. Econ. Bot. 1970, 24, 20–31. [Google Scholar] [CrossRef]
- Bedigian, D. Evolution of sesame revisited: Domestication, diversity and prospects. Gen. Res. Crop Evol. 2003, 50, 779–787. [Google Scholar]
- Bedigian, D. A New Combination for the Indian Progenitor of Sesame, Sesamum indicum (Pedaliaceae). Novon 2014, 23, 5–13. [Google Scholar] [CrossRef]
- Faostat. Food and Agriculture Organization Statistical Databases. 2016. Available online: http://faostat.fao.org/ (accessed on 13 July 2019).
- Islam, F.; Gill, R.A.; Ali, B.; Farooq, M.A.; Xu, L.; Najeeb, U.; Zhou, W. Sesame. In Breeding Oilseed Crops for Sustainable Production-Opportunities and Constraints; Gupta, S.K., Ed.; Elsevier: London, UK, 2016; pp. 135–147. [Google Scholar]
- Budowski, P.; Markley, K.S. The chemical and physiological properties of sesame oil. Chem. Rev. 1951, 48, 125–151. [Google Scholar] [CrossRef]
- Schmidt, Š.; Pokorny, J. Potential application of oilseeds as sources of antioxidants for food lipids—A review. Czech J. Food Sci. 2005, 23, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Pathak, N.; Rai, A.K.; Kumari, R.; Bhat, K.V. Value addition in sesame: A perspective on bioactive components for enhancing utility and profitability. Pharmacogn. Rev. 2014, 8, 147–155. [Google Scholar]
- Namiki, M. The chemistry and physiological functions of sesame. Food Rev. Int. 1995, 11, 281–329. [Google Scholar] [CrossRef]
- Namiki, M. Nutraceutical functions of sesame: A review. Crit. Rev. Food Sci. Nutr. 2007, 47, 651–673. [Google Scholar] [CrossRef] [PubMed]
- Hardwicke, J.E.; King, J.; Terrell, R.C. Synthesis of Sesamol Acetate and Sesamol. U.S. Patent No. 2,885,407, 5 May 1959. [Google Scholar]
- Markus, R.L. Process for Producing Sesamol. U.S. Patent No. 3,058,995, 16 October 1962. [Google Scholar]
- Forse, R.A.; Chavali, S.R. Sesamol Inhibitor of Delta-5-Desaturase Activity and Uses Therefor. U.S. Patent No. 2001/0031275, 18 October 2001. [Google Scholar]
- You, J.W.; Rho, H.S.; Kim, D.H.; Chang, I.S.; Lee, O.S. Sesamol Derivatives and Their Salts, the Process for Preparing the Same, and the Skin External Composition Containing the Same. U.S. Patent No. 7,943,599, 17 May 2011. [Google Scholar]
- Yamada, D.; Kato, M.; Ono, Y.; Tomimori, N.; Nishiumi, T.; Nakahara, K. Eine Lignanverbindung enthaltende Öl-in-Wasser-Emulsion und diese enthaltende Zusammensetzung. Eur. Patent Bull. 2007, 50, 191. [Google Scholar]
- Sok, D.E.; Cui, H.S.; Kim, M.R. Isolation and bioactivities of furfuran type lignan compounds from edible plants. Recent Pat. Food Nutr. Agric. 2009, 1, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kono, E.; Tanaka, R. Lignan Glycosylation Enzyme and Its Use. Jap. Patent JP 4667007, 21 January 2011. [Google Scholar]
- Yamada, D.; Kato, M.; Ono, Y.; Tomimori, N.; Nishiumi, T.; Nakahara, K. Oil-in-Water Emulsions Containing Lignan-Class Compounds and Compositions Containing the Same. U.S. Patent No. 8,685,455, 1 April 2014. [Google Scholar]
- Kojima, A.; Yausa, I.; Kiyomoto, K.; Omura, A. Composition for Promoting Collagen Production, for Promoting Elastin Production and/or for Promoting Keratocyte Migration and Usage Therefor. U.S. Patent No. 2016/0175280, 23 June 2016. [Google Scholar]
- Zhang, H.; Miao, H.; Wei, L.; Li, C.; Zhao, R.; Wang, C. Genetic analysis and QTL mapping of seed coat color in sesame (Sesamum indicum L.). PLoS ONE 2013, 8, e63898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dossa, K. A physical map of important QTLs, functional markers and genes available for sesame breeding programs. Physiol. Mol. Biol. Plants 2016, 22, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Dossa, K.; Li, D.; Wang, L.; Zheng, X.; Yu, J.; Wei, X. Dynamic transcriptome landscape of sesame (Sesamum indicum L.) under progressive drought and after re-watering. Genom. Data 2017, 11, 122–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhou, F.; Zhou, T.; Yang, Y.; Zhao, Y. Cytological characterization and molecular mapping of a novel recessive genic male sterility in sesame (Sesamum indicum L.). PLoS ONE 2018, 13, e0204034. [Google Scholar] [CrossRef] [PubMed]
- Dossa, K.; Li, D.; Yu, J.; Wang, L.; Zhang, Y.; You, J.; Zhou, R.; Mmadi, M.A.; Li, A.; Fonceka, D.; et al. The genetic basis of drought tolerance in the high oil crop Sesamum indicum. Plant Biotechnol. J. 2019, 17, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Gong, H.; Yu, J.; Liu, P.; Wang, L.; Zhang, Y.; Zhang, X. SesameFG: An integrated database for the functional genomics of sesame. Sci. Rep. 2017, 7, 2342. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Yu, S.; Tong, C.; Zhao, Y.; Liu, Y.; Song, C.; Zhang, Y.; Zhang, X.; Wang, Y.; Hua, W.; et al. Genome sequencing of the high oil crop sesame. Genome Biol. 2014, 15, R39. [Google Scholar] [CrossRef] [Green Version]
- Wu, K.; Liu, H.; Yang, M.; Tao, Y.; Ma, H.; Wu, W.; Zuo, Y.; Zhao, Y. High-density genetic map construction and QTLs analysis of grain yield-related traits in Sesame (Sesamum indicum L.) based on RAD-Seq techonology. BMC Plant Biol. 2014, 14, 274. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Liu, K.; Zhang, Y.; Feng, Q.; Wang, L.; Zhao, Y.; Li, D.; Zhao, Q.; Zhu, X.; Zhu, X.; et al. Genetic discovery for oil production and quality in sesame. Nat. Commun. 2015, 6, 8609. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xia, Q.; Zhang, Y.; Zhu, X.; Zhu, X.; Li, D.; Ni, X.; Gao, Y.; Xiang, H.; Wei, X.; et al. Updated sesame genome assembly and fine mapping of plant height and seed coat color QTLs using a new high-density genetic map. BMC Genom. 2016, 17, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uncu, A.O.; Frary, A.; Karlovsky, P.; Doganlar, S. High-throughput single nucleotide polymorphism (SNP) identification and mapping in the sesame (Sesamum indicum L.) genome with genotyping by sequencing (GBS) analysis. Mol. Breed. 2016, 36, 173. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Zhu, X.; Yu, J.; Wang, L.; Zhang, Y.; Li, D.; Zhou, R.; Zhang, X. Identification of sesame genomic variations from genome comparison of landrace and variety. Front. Plant Sci. 2016, 7, 1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, R.; Dossa, K.; Li, D.; Yu, J.; You, J.; Wei, X.; Zhang, X. Genome-wide association studies of 39 seed yield-related traits in sesame (Sesamum indicum L.). Int. J. Mol. Sci. 2018, 19, 2794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moss, G.P. Nomenclature of lignans and neolignans. Pure. Appl. Chem. 2000, 72, 1493–1523. [Google Scholar] [CrossRef]
- Wu, D.; Wang, X.-P.; Zhang, W. Sesamolin Exerts Anti-proliferative and apoptotic effect on human colorectal cancer cells via inhibition of JAK2/STAT3 signaling pathway. Cell Mol. Biol. 2019, 65, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Matsuzaki, Y.; Koyama, M.; Hitomi, T.; Kawanaka, M.; Enoki-Konishi, M.; Okuyama, Y.; Takayasu, J.; Nishino, H.; Nishikawa, A. Sesamin, a lignan of sesame, down-regulates cyclin D1 protein expression in human tumor cells. Cancer Sci. 2007, 98, 1447–1453. [Google Scholar] [CrossRef]
- Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Pandey, M.K.; Joy, B.; Guha, S.; Krishnan, S.; Aggarwal, B.B. Sesamin manifests chemopreventive effects through the suppression of NF-kappa B-regulated cell survival, proliferation, invasion, and angiogenic gene products. Mol. Cancer Res. 2010, 8, 751–761. [Google Scholar] [CrossRef] [Green Version]
- Srisayam, M.; Weerapreeyakul, N.; Kanokmedhakul, K. Inhibition of two stages of melanin synthesis by sesamol, sesamin and sesamolin. Asian Pac. J. Trop. Biomed. 2017, 7, 886–895. [Google Scholar] [CrossRef]
- Pecquet, C.; Leynadier, F.; Saiag, P. Immediate hypersensibility to sesame in foods and cosmetics. Contact Derm. 1998, 39, 313. [Google Scholar] [CrossRef]
- Landete, J.M. Plant, mammalian lignans: A review of source, intake, metabolism, intestinal bacteria, health. Food Res. Int. 2012, 46, 410–424. [Google Scholar] [CrossRef]
- Xu, W.-H.; Zhao, P.; Wang, M.; Liang, Q. Naturally occurring furofuran lignans: Structural diversity and biological activities. Nat. Prod. Res. 2019, 33, 1357–1373. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, C.; Sanchez-Quesada, C.; Toledo, E.; Delgado- Rodriguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [Green Version]
- Durazzo, A.; Lucarini, M.; Camilli, E.; Marconi, S.; Gabrielli, P.; Lisciani, S.; Gambelli, L.; Aguzzi, A.; Novellino, E.; Santini, A.; et al. Dietary lignans: Definition, description and research trends in databases development. Molecules 2018, 23, 3251. [Google Scholar] [CrossRef] [Green Version]
- Dar, A.A.; Arumugam, N. Lignans of sesame: Purification methods, biological activities and biosynthesis-a review. Bioorg. Chem. 2013, 50, 1–10. [Google Scholar] [CrossRef]
- Haworth, R.D. Natural resins. Annu. Rep. Prog. Chem. 1936, 33, 266. [Google Scholar]
- Tocher, J.F. Isolation of another substance from sesame oil. Am. J. Pharm. 1891, 63, 142–146. [Google Scholar]
- Tocher, J.F. Isolation of another substance from sesame oil. Pharm. J. Trans. 1891, 3, 639–640. [Google Scholar]
- Tocher, J.F. A further note on sesamin. Pharm. J. Trans. 1893, 4, 700–702. [Google Scholar]
- Bruchhausen, V.F.; Gerhard, H. Zur Konstitution des Asarinins. Ber. Dtsch. Chem. Ges. 1939, 72, 830–838. [Google Scholar] [CrossRef]
- Budowski, P.; O’Connor, R.T.; Field, E.T. Sesame oil VI. Determination of sesamin. J. Am. Oil Chem. Soc. 1951, 28, 51–54. [Google Scholar] [CrossRef]
- Freudenberg, K.; Sidhu, G.S. Die absolute konfiguration des sesamins und pinoresinols. Tetrahedron Lett. 1960, 1, 3–6. [Google Scholar] [CrossRef]
- Katsuzaki, H.; Osawa, T.; Kawakishi, S. Chemistry and antioxidative activity of lignan glucosides in sesame seed. ACS Symp. Ser. 1994, 574, 275–280. [Google Scholar]
- Ryu, S.; Ho, C.T.; Osawa, T. High performance liquid chromatographic determination of antioxidant lignan glycosides in some variations of sesame. J. Food Lipids 1998, 5, 17–28. [Google Scholar] [CrossRef]
- Moazzami, A.A.; Haese, S.L.; Karnal-Eldin, A. Lignan contents in sesame seeds and products. Eur. J. Lipid Sci. Tech. 2007, 109, 1022–1027. [Google Scholar] [CrossRef]
- Kim, A.Y.; Yun, C.I.; Lee, J.G.; Kim, Y.J. Determination and daily intake estimation of lignans in sesame seeds and sesame oil products in Korea. Foods 2020, 9, 394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.S.; Lee, J.R.; Lee, J.S. Determination of sesamin and sesamolin in sesame (Sesamum indicum L.) seeds using UV spectrophotometer and HPLC. Korean J. Crop Sci. 2006, 51, 95–100. [Google Scholar]
- Shyu, Y.S.; Hwang, L.S. Antioxidative activity of the crude extract of lignan glycosides from unroasted Burma black sesame meal. Food Res. Int. 2002, 35, 357–365. [Google Scholar] [CrossRef]
- Yasumoto, S.S.; Komeichi, M.; Okuyama, Y.; Horigane, A. A simplified HPLC quantification of sesamin and sesamolin in sesame seed. SABRAO J. Breed. Genet. 2003, 35, 27–34. [Google Scholar]
- Wang, L.; Zhang, Y.; Li, P.; Wang, X.; Zhang, W.; Wie, W.; Zhang, X. HPLC analysis of seed sesamin and sesamolin variation in a sesame germplasm collection in China. J. Am. Oil Chem. Soc. 2012, 89, 1011–1020. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Li, P.; Zhang, W.; Wang, X.; Qi, X.; Zhang, X. Variation of sesamin and sesamolin contents in sesame cultivars from China. Pak. J. Bot. 2013, 45, 177–182. [Google Scholar]
- Dar, A.A.; Kancharla, P.K.; Chandra, K.; Sodhi, Y.S.; Arumugam, N. Assessment of variability in lignan and fatty acid content in the germplasm of Sesamum indicum L. J. Food Sci. Technol. 2019, 56, 976–986. [Google Scholar] [CrossRef]
- Suarez, C.; O’Connor, R.T.; Field, E.T.; Bickford, W.G. Determination of sesamol, sesamolin, and sesamin in sesamin concentrates and oils. Anal. Chem. 1952, 24, 668–671. [Google Scholar] [CrossRef]
- Beroza, M. Determination of sesamin, sesamolin, and sesamol. Anal. Chem. 1954, 26, 1173–1176. [Google Scholar] [CrossRef]
- Dar, A.A.; Verma, N.K.; Arumugam, N. An updated method for isolation, purification and characterization of clinically important antioxidant lignans—Sesamin and sesamolin, from sesame oil. Ind. Crop. Prod. 2015, 64, 201–208. [Google Scholar] [CrossRef]
- Shi, L.K.; Liu, R.J.; Jin, Q.Z.; Wang, X.G. The contents of lignans in sesame seeds and commercial sesame oils of China. J. Am. Oil. Chem. Soc. 2017, 94, 1035–1044. [Google Scholar] [CrossRef]
- Bedigian, D.; Seigler, D.S.; Harlan, J.R. Sesamin, sesamolin and the origin of sesame. Biochem. Syst. Ecol. 1985, 13, 133–139. [Google Scholar] [CrossRef]
- Hemalatha, S.; Ghafoorunissa, H.S. Lignans and tocopherols in Indian sesame cultivars. J. Am. Oil Chem. Soc. 2004, 81, 467. [Google Scholar] [CrossRef]
- Canzoneri, F.; Perciabosco, F. Sulle sostanze clie accompagnano l’olio nei semi di sesamo. Gazz. Chim. Ital. 1903, 33, 253–260. [Google Scholar]
- Adriani, W. Über sesamin und sesamolin. Zeitschr. Unters. Lebensm. 1928, 56, 187–194. [Google Scholar] [CrossRef]
- Haslam, E.; Haworth, R.D. The constituents of natural phenolic resins. Part XXIII. The constitution of sesamolin. J. Chem. Soc. 1955, 71, 827–833. [Google Scholar] [CrossRef]
- Fukuda, Y.T.; Osawa, S.; Namiki, M.; Ozaki, T. Studies on antioxidative substances in sesame seed. Agric. Bio Chem. 1985, 49, 301–306. [Google Scholar]
- Fukuda, Y.T.; Osawa, S.; Kawagishi, L.; Namiki, M. Comparison of contents of sesamolin and lignan antioxidants in sesame seeds cultivated in Japan. Nippon Shokuhin Kogyo Gakkaishi 1988, 35, 483–486. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, Y.; Nagata, M.; Osawa, T.; Namiki, M. Contribution of lignan analogues to antioxidative activity of refined unroasted sesame seed oil. J. Am. Oil Chem. Soc. 1986, 63, 1027–1031. [Google Scholar] [CrossRef]
- Kumazawa, S.; Koike, M.; Usui, Y.; Nakayma, T.; Fukuda, Y. Isolation of sesaminols as antioxidative components from roasted sesame seed oil. J. Oleo Sci. 2003, 52, 303–307. [Google Scholar] [CrossRef]
- Osawa, T.; Nagata, M.; Namiki, M.; Fukuda, Y. Sesamolinol: A novel antioxidant isolated from sesame seeds. Agric. Biol. Chem. 1985, 49, 3351–3352. [Google Scholar]
- Katsuzaki, H.; Kawasumi, M.; Kawakishi, S.; Osawa, T. Structure of novel antioxidative lignan glucosides isolated from sesame seed. Biosci. Biotechnol. Biochem. 1992, 56, 2087–2088. [Google Scholar] [CrossRef]
- Milder, I.E.J.; Arts, I.C.W.; van de Putte, B.; Venema, D.P.; Hollman, P.C.H. Lignan contents of Dutch plant foods: A database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br. J. Nutr. 2005, 93, 393–402. [Google Scholar] [CrossRef]
- Roy, S.C.; Rana, K.K.; Guin, C. Short and stereoselective total synthesis of furano lignans (±)-dihydrosesamin, (±)-lariciresinol dimethyl ether, (±)-acuminatin methyl ether, (±)-sanshodiol methyl ether, (±)-lariciresinol, (±)-acuminatin, and (±)-lariciresinol monomethyl ether and furofuran lignans (±)-sesamin, (±)-eudesmin, (±)-piperitol methyl ether, (±)-pinoresinol, (±)-piperitol, and (±)-pinoresinol monomethyl ether by radical cyclization of epoxides using a transition-metal radical source. J. Org. Chem. 2002, 67, 3242–3248. [Google Scholar]
- Malagnini, G.; Armanni, G. Ricerche sull’olio di sesamo. Rend. Soc. Chim. Roma 1907, 5, 133–137. [Google Scholar]
- Malagnini, G.; Armanni, G. Untersuchungen über Sesamöl. Chem. Ztg. 1907, 31, 884–885. [Google Scholar]
- Budowski, P.; O’Connor, R.T.; Field, E.T. Sesame oil 4. Determination of free and bound sesamol. J. Am. Oil Chem. Soc. 1950, 27, 307–310. [Google Scholar] [CrossRef]
- Umezawa, T.; Shimada, M. Syntheses of (±)-lariciresinols. Mokuzai Gakkaishi 1994, 40, 231–235. [Google Scholar]
- Michailidis, D.; Angelis, A.; Aligiannis, N.; Mitakou, S.; Skaltsounis, L. Recovery of sesamin, sesamolin, and minor lignans from sesame oil using solid support-free liquid–liquid extraction and chromatography techniques and evaluation of their enzymatic inhibition properties. Front. Pharmacol. 2019, 10, 723. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.Y.; Lee, W.J.; Chu, I.H.; Hung, W.C.; Su, N.W. Formation of samin diastereomers by acid-catalyzed transformation of sesamolin with hydrogen peroxide. J. Agric. Food Chem. 2020, 68, 6430–6438. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Nakai, M.; Fukui, Y.; Tomimori, N.; Fukuchi-Mizutani, M.; Saito, M.; Satake, H.; Tanaka, T.; Katsuta, M.; Umezawa, T.; et al. Formation of two methylenedioxy bridges by a Sesamum CYP81Q proten yielding a furofuran lignan, (+)-sesamin. Proc. Natl. Acad. Sci. USA 2006, 103, 10116–10121. [Google Scholar] [CrossRef] [Green Version]
- Marchand, P.A.; Kato, M.J.; Lewis, N.G. (+)-Episesaminone, a Sesamum indicum furofuran lignan. Isolation and hemisynthesis. J. Nat. Prod. 1997, 60, 1189–1192. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.Å. Variations in the composition of sterols, tocopherols and lignans in seed oils from four Sesamum species. J. Am. Oil Chem. Soc. 1994, 71, 149–156. [Google Scholar] [CrossRef]
- Ryu, S.R.; Kang, S.S.; Lee, J.I.; Choi, C.Y. Isolation and quantitative analysis of sesangolin in wild sesame. Korean J. Breed. 1993, 25, 55–58. [Google Scholar]
- Ono, E.; Murata, J.; Toyonaga, H.; Nakayasu, M.; Mizutani, M.; Yamamoto, M.P.; Umezawa, T.; Horikawa, M. Formation of a methylenedioxy bridge in (+)-Epipinoresinol by CYP81Q3 corroborates with diasteremeric specialization in sesame lignans. Plant Cell Physiol. 2018, 59, 2278–2287. [Google Scholar]
- Kamal-Eldin, A.; Yousif, G. A furofuran lignan from Sesamum alatum. Phytochemistry 1992, 31, 2911–2912. [Google Scholar] [CrossRef]
- Jones, W.; Beroza, M.; Becker, E. Isolation and structure of sesangolin, a constituent of Sesamum angolense. J. Org. Chem. 1962, 27, 3232–3235. [Google Scholar] [CrossRef]
- Harada, E.; Murata, J.; Ono, E.; Toyonaga, H.; Shiraishi, A.; Hideshima, K.; Yamamoto, M.P.; Horikawa, M. (+)-Sesamin-oxidising CYP92B14 shapes specialised lignan metabolism in sesame. Plant J. 2020, 104, 1117–1128. [Google Scholar] [CrossRef]
- Pathak, N.; Bhaduri, A.; Bhat, K.V.; Rai, A.K. Tracking sesamin synthase gene expression through seed maturity in wild and cultivated sesame species—A domestication footprint. Plant Biol. 2015, 17, 1039–1046. [Google Scholar] [CrossRef]
- Pathak, N.; Rai, A.K.; Saha, S.; Walia, S.; Sen, S.K.; Bhat, K.V. Quantitative dissection of antioxidative bioactive components in cultivated and wild sesame germplasm reveals potentially exploitable wide genetic variability. J. Crop Sci. Biotechnol. 2014, 17, 127–139. [Google Scholar] [CrossRef]
- Kamal-Eldin, A. Chemical studies on the lignans and other minor constituents of sesame seed oil. In Sesame—The genus Sesamum; Bedigian, D., Ed.; CRC Press, Taylor and Francis Group: Abingdon, UK, 2010; 560p. [Google Scholar]
- Grougnet, R.; Magiatis, P.; Mitaku, S.; Terzis, A.; Tillequin, F.; Skaltsounis, A.L. New lignans from the perisperm of Sesamum indicum. J. Agric. Food Chem. 2006, 54, 7570–7574. [Google Scholar] [CrossRef]
- Weinges, K. Die Lignane des Überwallungsharzes der Fichte. Tetrahedron Lett. 1960, 1, 1–2. [Google Scholar] [CrossRef]
- Katsuzaki, H.; Kawakishi, S.; Osawa, T. Sesaminol glucosides in sesame seeds. Phytochemistry 1994, 35, 773–776. [Google Scholar] [CrossRef]
- Nagata, M.; Osawa, T.; Namiki, M.; Fukuda, Y. Stereochemical structures of antioxidative sesaminol. Agric. Biol. Chem. 1987, 51, 1285–1289. [Google Scholar]
- Moazzami, A.A.; Andersson, R.E.; Kamal-Eldin, A. Characterization and analysis of sesamolinol diglucoside in sesame seeds. Biosci. Biotechnol. Biochem. 2006, 70, 1478–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moazzami, A.A.; Kamal-Eldin, A. Sesame seed is a rich source of dietary lignans. J. Am. Oil. Chem. Soc. 2006, 83, 719–723. [Google Scholar] [CrossRef]
- Moazzami, A.A.; Andersson, R.E.; Kamal-Eldin, A. HPLC analysis of sesaminol glucosides in sesame seeds. J. Agric. Food Chem. 2006, 54, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; Bhaduri, A.; Rai, A. Sesame: Bioactive compounds and health benefits. In Biaocative Molecules in Food; Merillon, J.M., Ramawat, K., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2017; pp. 1–20. [Google Scholar]
- Gerstenmeyer, E.; Reimer, S.; Berghofer, E.; Schwartz, H.; Sontag, G. Effect of thermal heating on some lignans in flax seeds, sesame seeds and rye. Food Chem. 2013, 138, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Tokar, M.; Klimek, B. The content of lignan glycosides in Forsythia flowers and leaves. Acta Pol. Pharm. 2004, 61, 273–278. [Google Scholar]
- Youssef, F.S.; Ashour, M.L.; El-Beshbishy, H.A.; Hamza, A.A.; Singab, A.N.B.; Wink, M. Pinoresinol-4-O-β-d-glucopyranoside: A lignan from prunes (Prunus domestica) attenuates oxidative stress, hyperglycaemia and hepatic toxicity in vitro and in vivo. J. Pharm. Pharmacol. 2020, 72, 1830–1839. [Google Scholar] [CrossRef]
- Zhang, Z.-F.; Min, J.-K.; Wang, D.; Zhong, J.-M. Pinoresinol diglucoside exhibits protective effect on dexamethasone-induced osteoporosis in rats. Trop. J. Pharm. Res. 2016, 15, 2451–2457. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.H.; Akao, T.; Hamasaki, K.; Deyama, T.; Hattori, M. Biotransformation of pinoresinol diglucoside to mammalian lignans by human intestinal microflora, and isolation of Enterococcus faecalis strain PDG-1 responsible for the transformation of (+)-pinoresinol to (+)-lariciresinol. Chem. Pharm. Bull. 2003, 51, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Zou, Z.; Wang, X.; Ji, X.; Yang, J. Pinoresinol diglucoside alleviates oxLDL-induced dysfunction in human umbilical vein endothelial cells. Evid. Based Complement. Alternat. Med. 2016, 2016, 3124519. [Google Scholar] [CrossRef]
- Song, J.Z.; Cheung, L.M.; Liu, X.; Qiao, C.F.; Zhou, Y.; Li, S.L.; Chen, S.L.; Xu, H.X. Development and validation of an ultra high-performance liquid chromatographic method for the determination of a diastereomeric impurity in (+)-pinoresinol diglucoside chemical reference substance. J. Sep. Sci. 2010, 33, 1909–1915. [Google Scholar] [CrossRef]
- Milder, I.E.J.; Arts, I.C.W.; Venema, D.P.; Lasaroms, J.J.P.; Wähälä, K.; Hollman, P.C.H. Optimization of a liquid chromatography—tandem mass spectrometry method for quantification of the plant lignans secoisolariciresinol, matairesinol, lariciresinol, and pinoresinol in foods. J. Agric. Food Chem. 2004, 52, 4643–4651. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Lin, M.; Lin, P.; Chen, J. Effects of thermal preparation and in vitro digestion on lignan profiles and antioxidant activity in defatted-sesame meal. Food Chem. Toxicol. 2019, 128, 89–96. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Lin, M.; Zheng, Y.; Chen, J. Effect of roasting and in vitro digestion on phenolic profiles and antioxidant activity of water-soluble extracts from sesame. Food Chem. Toxicol. 2020, 139, 111239. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Guignard, C.; Renaut, J.; Hausman, J.-F.; Gatti, E.; Predieri, S.; Guerriero, G. Insights into Lignan Composition and Biosynthesis in Stinging Nettle (Urtica dioica L.). Molecules 2019, 24, 3863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deyama, T. The Constituents of Eucommia ulmoides OLIV. I. Isolation of (+)-medioresinol di-O-β-d-glucopyranoside. Chem. Pharm. Bull. 1983, 31, 2993–2997. [Google Scholar] [CrossRef] [Green Version]
- Weiss, F.E. A Gutta-percha plant. Nature 1899, 61, 7. [Google Scholar] [CrossRef] [Green Version]
- Parkin, J. Eucommia Ulmoides. The Tu-Chung of the Chinese. Bull. Misc. Inform. Kew 1921, 1921, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Kreis, H. Über neue Farbreaktionen fetter Öle. Chem. Ztg. 1903, 27, 1030–1033. [Google Scholar]
- Villavecchia, V.; Fabris, G. Über die Anwendung des Furfurols als Reagens zur Erkennung des Sesamöls in Ölmischungen. Z. Angew. Chem. 1893, 6, 505–506. [Google Scholar] [CrossRef] [Green Version]
- Yen, G.C. Influence of seed roasting process on the changes in composition and quality of sesame (Sesame indicum) oil. J. Sci. Food Agric. 1990, 50, 563–570. [Google Scholar] [CrossRef]
- Yoshida, H. Composition and quality characteristics of sesame seed (Sesamum indicum) oil roasted at different temperatures in an electric oven. J. Sci. Food Agric. 1994, 65, 331–336. [Google Scholar] [CrossRef]
- Honig, P. Over veranderingen in de sesam-oliereactie bij behandeling der olie met adsorbentia. Chem. Weekbl. 1925, 22, 509–512. [Google Scholar]
- Fukuda, Y.; Nagata, M.; Osawa, T.; Namiki, M. Chemical aspects of the antioxidative activity of roasted sesame seed oil and the effect of using the oil for frying. Agric. Biol. Chem. 1986, 50, 857–862. [Google Scholar]
- Wanasundara, P.K.J.P.D.; Shahidi, F. Process-Induced Chemical Changes in Food. In Process-Induced Changes in Edible Oils; Shahidi, F., Ho, C.T., Chuyen, N.V., Eds.; Plenum Publishers: New York, NY, USA, 1998; pp. 135–160. [Google Scholar]
- Kim, I.; Choe, E. Effects of bleaching on the properties of roasted sesame oil. J. Food Sci. 2005, 70, 48–52. [Google Scholar] [CrossRef]
- Yoshida, H.; Takagi, S. Effects of seed roasting temperature and time on the quality characteristics of sesame (Sesamum indicum) oil. J. Sci. Food Agric. 1997, 75, 19–26. [Google Scholar] [CrossRef]
- Kikugawa, K.; Arai, M.; Kurechi, T. Participation of sesamol in stability of sesame oil. J. Am. Oil. Chem. Soc. 1983, 60, 1528–1533. [Google Scholar] [CrossRef]
- Masuda, T.; Shingai, Y.; Fujimoto, A.; Nakamura, M.; Oyama, Y.; Maekawa, T.; Sone, Y. Identification of cytotoxic dimers in oxidation product from sesamol, a potent antioxidant of sesame oil. J. Agric. Food Chem. 2010, 58, 10880–10885. [Google Scholar] [CrossRef]
- Shingai, Y.; Fujimoto, A.; Nakajima, A.; Saito, M.; Kanemaru, K.; Masuda, T.; Oyama, Y. Cytotoxic characteristics of two isomeric dimers produced by oxidation of sesamol, an antioxidant in sesame oil. J. Health Sci. 2011, 57, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Aoshima, Y.; Nakai, M.; Katano, K.; Okada, A. Process and an Apparatus for Producing Episesamin-Rich Compositions. U.S. Patent No. 7,943,663, 17 May 2011. [Google Scholar]
- Fukuda, Y.; Isobe, M.; Nagata, M.; Osawa, T.; Namiki, M. Acidic transformation of sesamolin of sesame oil constituent into an antioxidant bisepoxylignan, sesaminol. Heterocycles 1986, 24, 923–926. [Google Scholar] [CrossRef]
- Huang, J.; Song, G.; Zhang, L.; Sun, Q.; Lu, X. A novel conversion of sesamolin to sesaminol by acidic cathion resin. Eur. J. Lipid Sci. Technol. 2012, 114, 842–848. [Google Scholar] [CrossRef]
- Jan, K.C.; Hwang, L.S.; Ho, C.T. Tissue distribution and elimination of sesaminol triglucoside and its metabolites in rat. Mol. Nutr. Food Res. 2009, 53, 815–825. [Google Scholar] [CrossRef]
- Jan, K.C.; Hwang, L.S.; Ho, C.T. Biotransformation of sesaminol triglucoside to mammalian lignans by intestinal microbiota. J. Agric. Food Chem. 2009, 57, 6101–6106. [Google Scholar] [CrossRef]
- Kuriyama, K.; Tsuchiya, K.; Murui, T. Analysis of lignan glycosides in sesame seed by high-pressure liquid chromatography. Nippon Nōgeikagaku Kaishi 1993, 67, 1693–1700. [Google Scholar] [CrossRef]
- Park, S.H.; Ryu, S.N.; Bu, Y.; Kim, H.; Simon, J.E.; Kim, K.S. Antioxidant components as potential neuroprotective agents in sesame (Sesamum indicum L.). Food Rev. Int. 2010, 26, 103–121. [Google Scholar] [CrossRef]
- Peng, Z.; Xu, Y.; Meng, Q.; Raza, H.; Zhao, X.; Liu, B.; Dong, C. Preparation of sesaminol from sesaminol triglucoside by β-glucosidase and cellulase hydrolysis. J. Am. Oil Chem. Soc. 2016, 93, 765–772. [Google Scholar] [CrossRef]
- Nair, A.; Kuwahara, A.; Nagase, A.; Yamaguchi, H.; Yamazaki, T.; Hosoya, M.; Omura, A.; Kiyomoto, K.; Yamaguchi, M.; Shimoyama, T.; et al. Purification, gene cloning, and biochemical characterization of a β-glucosidase capable of hydrolyzing sesaminol triglucoside from Paenibacillus sp. KB0549. PLoS ONE 2013, 8, e60538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.; Nakayama, T.; Kiyomoto, K. Sesaminol Glycoside Glucoside Bond Degrading Enzyme and Microorganism Producing Said Enzyme. Japanese Patent Aplication No. 4839231, 21 December 2011. (In Japanese). [Google Scholar]
- Sakurai, A.; Hongo, S.; Nair, A.; Waki, T.; Oikawa, D.; Nishio, T.; Shimoyama, T.; Takahashi, S.; Yamashita, S.; Nakayama, T. Identification and characterization of a novel bacterial β-glucosidase that is highly specific for the β-1,2-glucosidic linkage of sesaminol triglucoside. Biosci. Biotechnol. Biochem. 2018, 82, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Gaya, P.; Peirotén, Á.; Landete, J.M. Expression of a β-glucosidase in bacteria with biotechnological interest confers them the ability to deglycosylate lignans and flavonoids in vegetal foods. Appl. Microbiol. Biotechnol. 2020, 104, 4903–4913. [Google Scholar] [CrossRef] [PubMed]
- Kamal-Eldin, A.; Appelqvist, L.Å.; Yousif, G. Lignan analysis in seed oils from four sesamum species: Comparison of different chromatographic methods. J. Am. Oil Chem. Soc. 1994, 71, 141–147. [Google Scholar] [CrossRef]
- Katekhaye, S.; Gavit, R.; Laddha, K. A simple method for isolation of sesamin from Sesamum indicum Linn. seed oil. Indian Drugs 2011, 48, 54–58. [Google Scholar]
- Corona, G.; Kreimes, A.; Barone, M.; Turroni, S.; Brigidi, P.; Keleszade, E.; Costabile, A. Impact of lignans in oilseed mix on gut microbiome composition and enterolignan production in younger healthy and premenopausal women: An in vitro pilot study. Microb. Cell Factories 2020, 19, 82. [Google Scholar] [CrossRef] [Green Version]
- Westcott, N.D.; Muir, A.D. Process for Extracting Lignans from Flaxseed. U.S. Patent No. 5,705,618, 6 January 1998. [Google Scholar]
- Raffaelli, B.; Hoikkala, A.; Wähälä, A. Enterolignans. J. Chromatogr. B 2002, 777, 29–43. [Google Scholar] [CrossRef]
- Penalvo, J.; Heinonen, S.; Aura, A.; Adlercreutz, H. Dietary sesamin is converted to enterolactone in humans. J. Nutr. 2005, 135, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Borriello, S.P.; Setchell, K.D.R.; Axelson, M.; Lawson, A.M. Production and metabolism of lignans by the human fecal flora. J. Appl. Bacteriol. 1985, 58, 37–43. [Google Scholar] [CrossRef]
- Wang, L. Mammmalian phytoestrogens: Enterodiol and enterolactone. J. Chromatogr. B Biomed. Appl. 2002, 777, 289–309. [Google Scholar] [CrossRef]
- Clavel, T.; Henderson, G.; Alpert, C.A.; Philippe, C.; Rigottier-Gois, L.; Dore, J.; Blaut, M. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl. Environ. Microbiol. 2005, 71, 6077–6085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.Q.; Meselhy, M.R.; Li, Y.; Qin, G.W.; Hattori, M. Human intestinal bacteria capable of transforming secoisolariciresinol diglucoside to mammalian lignans, enterodiol and enterolactone. Chem. Pharm. Bull. 2000, 48, 1606–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Saarinen, N.M.; Thompson, L.U. Sesamin is one of the major precursors of mammalian lignans in sesame seed (Sesamum indicum) as observed in vitro and in rats. J. Nutr. 2006, 136, 906–912. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.-S.; Zhao, Y.-F.; Nakamura, N.; Akao, T.; Kakiuchi, N.; Min, B.-S.; Hattori, M. Enantioselective dehydroxylation of enterodiol and enterolactone precursors by human intestinal bacteria. Biol. Pharm. Bull. 2007, 30, 2113–2119. [Google Scholar] [CrossRef] [Green Version]
- Clavel, T.; Henderson, G.; Engst, W.; Doré, J.; Blaut, M. Phylogeny of human intestinal bacteria that activate the dietary lignan secoisolariciresinol diglucoside. FEMS Microbiol. Ecol. 2006, 55, 471–478. [Google Scholar] [CrossRef]
- Jin, J.S.; Hattori, M. Further studies on a human intestinal bacterium Ruminococcus sp. END-1 for transformation of plant lignans to mammalian lignans. J. Agric. Food Chem. 2009, 57, 7537–7542. [Google Scholar] [CrossRef]
- Nakai, M.; Harada, M.; Nakahara, K.; Akimoto, K.; Shibata, H.; Miki, W.; Kiso, Y. Novel antioxidative metabolites in rat liver with ingested sesamin. J. Agric. Food Chem. 2003, 51, 1666–1670. [Google Scholar] [CrossRef]
- Yasuda, K.; Ikushiro, S.; Kamakura, M.; Ohta, M.; Sakaki, T. Metabolism of sesamin by cytochrome P450 in human liver microsomes. Drug Metab. Dispos. 2010, 38, 2117–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakaki, T.; Yasuda, K.; Kamakura, M.; Munetsuna, E.; Ohta, M.; Ikushiro, S. Metabolism of sesamin by CYPs and UGTs in human liver. J. Food Drug. Anal. 2012, 20, 376–379. [Google Scholar] [CrossRef]
- Moazzami, A.A.; Anderson, R.E.; Kamal-Eldin, A. Quantitative NMR analysis of a sesamin catechol metabolite in human urine. J. Nutr. 2007, 137, 940–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiroto, T.; Oka, K. Method of Producing Sesamol Formic Acid Ester and Sesamol. European Patent Specification EP 1 167365, 22 October 2003. [Google Scholar]
- Mori, N.; Furuta, A.; Watanabe, H. Electochemical asymmetric dimerization of cinnamic acid derivatives and application to the enantioselective syntheses of furofuran lignans. Tetrahedron 2016, 72, 8393–8399. [Google Scholar] [CrossRef]
- Albertson, A.K.F.; Lumb, J.P. A bio-inspired total synthesis of tetrahydrofuran lignans. Angew. Chem. Int. Ed. 2015, 54, 2204–2208. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, R.; Yoshida, N.; Akao, Y.; Kawabe, Y.; Inai, M.; Asakawa, T.; Hamashima, Y.; Kan, T. Total syntheses of (+)-sesamin and (+)-sesaminol. Chem. Lett. 2014, 43, 1572–1574. [Google Scholar] [CrossRef]
- Pohmakotr, M.; Kuhakarn, C.; Reutrakul, V.; Soorukram, D. Asymmetric synthesis of furofurans. Tetrahedron Lett. 2017, 58, 4740–4746. [Google Scholar] [CrossRef]
- Soorukram, D.; Pohmakotr, M.; Kuhakarn, C.; Reutrakul, V. Stereoselective synthesis of tetrahydrofuran lignans. Synthesis 2018, 50, 4746–4764. [Google Scholar] [CrossRef]
- Fang, X.; Hu, X. Advances in the synthesis of lignan natural products. Molecules 2018, 23, 3385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.Y.; Jeong, H.W.; Seo, D.B.; Lee, S.J. Method for Preparing Furofuran Lignan Compound. U.S. Patent Application No. 2015/0073158, 12 March 2015. [Google Scholar]
- Hemalatha, S.; Ghafoorunissa, H. Sesame lignans enhance the thermal stability of edible vegetable oils. Food Chem. 2007, 105, 1076–1085. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Choe, E. Antioxidant activity of lignin compounds extracted from roasted sesame oil on the oxidation of sunflower oil. Food Sci. Biotechnol. 2007, 16, 981–987. [Google Scholar]
- Hussain, S.A.; Hameed, A.; Ajmal, I.; Nosheen, S.; Suleria, H.A.R.; Song, Y. Effects of sesame seed extract as a natural antioxidant on the oxidative stability of sunflower oil. J. Food Sci. Technol. 2018, 55, 4099–4110. [Google Scholar] [CrossRef]
- Newton, R.C.; Grettie, D.P. Antioxidant for Fats and Oils. U.S. Patent No. 1,903,126, 28 March 1933. [Google Scholar]
- Alderks, O.H.; Distel, W.R.; Taylor, J.E. Edible Fat. U.S. Patent No. 1,985,969, 1 January 1935. [Google Scholar]
- Brown, L.C.; Grettie, D.P.; Newton, R.C. Baking Fat. British Patent No. 458,581, 14 December 1936. [Google Scholar]
- Silkeberg, A.; Kochhar, S.P. Refining of Edible Oil Retaining Maximum Antioxidative Potency. U.S. Patent No. 6,033,706, 7 March 2000. [Google Scholar]
- Górnaś, P.; Siger, A.; Pugajeva, I.; Segliņa, D. Sesamin and sesamolin as unexpected contaminants in various cold-pressed plant oils: NP-HPLC/FLD/DAD and RP-UPLC-ESI/MS study. Food Addit. Contam. Part A 2014, 31, 567–573. [Google Scholar] [CrossRef]
- Shukla, A.K.; Dixit, A.K.; Singh, R.P. Detection of adulteration in edible oils. J. Oleo Sci. 2005, 54, 317–324. [Google Scholar] [CrossRef]
- Joshi, S. FSSAI issues notice calling for removing Boudouin test needed for veg oil. India’s First F & B News, 24 July 2017. [Google Scholar]
- Jeon, J.S.; Park, C.L.; Syed, A.S.; Kim, Y.M.; Cho, I.J.; Kim, C.Y. Preparative separation of sesamin and sesamolin from defatted sesame meal via centrifugal partition chromatography with consecutive sample injection. J. Chromatogr. B 2016, 1011, 108–113. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.Å. Variation in fatty acid composition of the different acyl lipids in seed oils from four Sesamum species. J. Am. Oil Chem. Soc. 1994, 71, 135–139. [Google Scholar] [CrossRef]
- Bhatnagar, A.S.; Hemavathy, J.; Gopala Krishna, A.G. Development of a rapid method for determination of lignans content in sesame oil. J. Food Sci. Technol. 2015, 52, 521–527. [Google Scholar] [CrossRef]
- Wikul, A.; Damsud, T.; Kataoka, K.; Phuwapraisirisan, P. (+)-Pinoresinol is a putative hypoglycemic agent in defatted sesame (Sesamum indicum) seeds though inhibiting α-glucosidase. Bioorg. Med. Chem. Lett. 2012, 22, 5215–5217. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Shahidi, F.; Pegg, R.B. Application of semipreparative RP-18HPLC for the purification of sesamin and sesamolin. J. Food Lipids 2001, 8, 85–94. [Google Scholar] [CrossRef]
- Elleuch, M.; Besbes, S.; Roiseux, O.; Blecker, C.; Attia, H. Quality characteristics of sesame seeds and by-products. Food Chem. 2007, 103, 641–650. [Google Scholar] [CrossRef]
- Smeds, A.I.; Eklund, P.C.; Sjöholm, R.E.; Willför, S.M.; Nishibe, S.; Deyama, T.; Holmbom, B.R. Quantification of a broad spectrum of lignans in cereals, oilseeds, and nuts. J. Agric. Food Chem. 2007, 55, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Khuimphukhieo, I.; Khaengkhan, P. Combining ability and heterosis of sesamin and sesamolin content in sesame. SABRAO J. Breed. Genet. 2018, 50, 180–191. [Google Scholar]
- Begum, A.N.; Nicolle, C.; Mila, I.; Lapierre, C.; Nagano, K.; Fukushima, K.; Heinonen, S.-M.; Adlercreutz, H.; Rémésy, C.; Scalbert, A. Dietary lignins are precursors of mammalian lignans in rats. J. Nutr. 2004, 134, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gravenhorst, C.O. Observations on the Baudouins Test. Ind. Eng. Chem. 1924, 16, 47–48. [Google Scholar] [CrossRef]
- Jamieson, G.S.; Baughman, W.F. The chemical composition of sesame oil. J. Frankl. Inst. 1924, 197, 840. [Google Scholar] [CrossRef]
- AOCS. Official and Tentative Methods, 2nd ed.; American Oil Chemists Society: Chicago, IL, USA, 1949. [Google Scholar]
- Lee, W.J.; Su, N.W.; Lee, M.H.; Lin, J.T. Examination of the modified Villavecchia test for detecting sesame oil. J. Am. Oil Chem. Soc. 2013, 90, 667–674. [Google Scholar] [CrossRef]
- Barr, M. Identification Test for Highly Refined Sesame Oil. U.S. Patent No. 5,811,313, 22 September 1998. [Google Scholar]
- Kamal-Eldin, A.; Yousif, G.; Appelqvist, L.Å. Thin-layer chromatographic separations of seed oil unsaponifiables from four sesamum species. J. Am. Oil Chem. Soc. 1991, 68, 844–847. [Google Scholar] [CrossRef]
- Sukumar, D.; Arimboor, R.; Arumughan, C. HPTLC fingerprinting and quantification of lignans as markers in sesame oil and its polyherbal formulations. J. Pharm. Biomed. Anal. 2008, 47, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Schwertner, H.A.; Rios, D.C. Analysis of sesamin, asarinin, and sesamolin by HPLC with photodiode and fluorescent detection and by GC/MS: Application to sesame oil and serum samples. J. Am. Oil Chem. Soc. 2012, 89, 1943–1950. [Google Scholar] [CrossRef]
- Shi, L.K.; Zheng, L.; Xiang, Y.F.; Liu, R.J.; Chang, M.; Jin, Q.Z.; Wang, X.G. A rapid method for simultaneous analysis of lignan and γ-tocopherol in sesame oil by using normal-phase liquid chromatography. J. Am. Oil Chem. Soc. 2018, 95, 13–19. [Google Scholar] [CrossRef]
- Takahashi, M.; Nishizaki, Y.; Sugimoto, N.; Takeuchi, H.; Nakagawa, K.; Akiyama, H.; Sato, K.; Inoue, K. Determination and purification of sesamin and sesamolin in sesame seed oil unsaponified matter using reversed-phase liquid chromatography coupled with photodiode array and tandem mass spectrometry and high-speed countercurrent chromatography. J. Sep. Sci. 2016, 39, 3898–3905. [Google Scholar] [CrossRef]
- Yan, G.; Li, Q.; Tan, H.; Ge, T. Electrospray ionization ion-trap time-of-flight tandem mass spectrometry of two furofurans: Sesamin and gmelinol. Rapid Commun. Mass Spectrom. 2007, 21, 3613–3620. [Google Scholar] [CrossRef]
- Morawska, K.; Festinger, N.; Chwatko, G.; Głowacki, R.; Ciesielski, W.; Smarzewska, S. Rapid electroanalytical procedure for sesamol determination in real samples. Food Chem. 2020, 309, 125789. [Google Scholar] [CrossRef]
- Aslışen, B.; Koçak, Ç.C.; Koçak, S. Electrochemical determination of sesamol in foods by square wave voltammetry at a boron-doped diamond electrode. Anal. Lett. 2020, 53, 343–354. [Google Scholar] [CrossRef]
- Soliman, M.A.; El-Sawy, A.A.; Fadel, H.M.; Osman, F. Effect of antioxidants on the volatiles of roasted sesame seeds. J. Agric. Food Chem. 1985, 33, 523–528. [Google Scholar] [CrossRef]
- Dachtler, M.; van de Put, F.H.M.; Stijn, F.V.; Beindorff, C.M.; Fritsche, J. On-line LC-NMR-MS characterization of sesame oil extracts and assessment of their antioxidant activity. Eur. J. Lipid Sci. Technol. 2003, 105, 488–496. [Google Scholar] [CrossRef]
- Kuo, P.C.; Lin, M.C.; Chen, G.F.; Yiu, T.J.; Tzen, J.T.C. Identification of methanol-soluble compounds in sesame and evaluation of antioxidant potential of its lignans. J. Agric. Food Chem. 2011, 59, 3214–3219. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Kao, Z.-H.; Lee, S.-W.; Wu, S.-N. Effects of sesamin, the major furofuran lignan of sesame oil, on the amplitude and gating of voltage-gated Na+ and K+ currents. Molecules 2020, 25, 3062. [Google Scholar] [CrossRef] [PubMed]
- Reshma, M.V.; Balachandran, C.; Arumughan, C.; Sunderasan, A.; Sukumaran, D.; Thomas, S.; Saritha, S.S. Extraction, separation and characterisation of sesame oil lignan for nutraceutical applications. Food Chem. 2010, 120, 1041–1046. [Google Scholar] [CrossRef]
- Lim, J.S.; Adachi, Y.; Takahashi, Y.; Ide, T. Comparative analysis of sesame lignans (sesamin and sesamolin) in affecting hepatic fatty acid metabolism in rats. Br. J. Nutr. 2007, 97, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolova, B.; Velikova, R.; Kuleva, L. Quantitative TLC for determination of the triacylglycerol composition of sesame seeds. J. Liq. Chromatogr. Relat. Technol. 2002, 25, 10–11. [Google Scholar] [CrossRef]
- Kumar, C.M.; Singh, S.A. Bioactive lignans from sesame (Sesamum indicum L.): Evaluation of their antioxidant and antibacterial effects for food applications. J. Food Sci. Technol. 2015, 52, 2934–2941. [Google Scholar] [CrossRef] [Green Version]
- Williamson, K.S.; Morris, J.B.; Pye, Q.N.; Kamat, C.D.; Hensley, K. A Survey of Sesamin and Composition of Tocopherol Variability from Seeds of Eleven Diverse Sesame (Sesamum Indicum L.) Genotypes Using HPLC-PAD-ECD. Phytochem. Anal. 2008, 19, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Tracy, R.L. Process for Extracting Pyrethrin Synergists from Sesame Oil. U.S. Patent No. 2,837,534, 3 June 1958. [Google Scholar]
- Namiki, M.; Kobayashi, T.; Hara, H. Process of Producing Sesame Lignans and/or Sesame Flavors. U.S. Patent No. 6,278,005, 21 August 2001. [Google Scholar]
- Hu, H.; Liu, R.; Liu, S.; Wang, S. Method for Extracting High-Content Sesame Lignan Oil from Sesame. Chinese Patent No. 104277909, 29 October 2014. (In Chinese). [Google Scholar]
- Yasumoto, S.; Katsuta, M. Breeding a high lignan content sesame cultivar in the prospect of promoting metabolic functionality. Jpn. Agric. Res. Q. 2006, 40, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Usman, S.M.; Viswanathan, P.; Manonmani, S.; Uma, D. Genetic studies on sesamin and sesamolin content and other yield attributing characters in sesame (Sesamum indicum L.). El. J. Plant Breed. 2020, 11, 132–138. [Google Scholar]
- Tashiro, T.; Fukuda, Y.; Osawa, T.; Namiki, M. Oil and minor components of sesame (Sesamum indicum L.) strains. J. Am. Oil Chem. Soc. 1990, 67, 506–511. [Google Scholar] [CrossRef]
- Bedigian, D. Sesame: The Genus Sesamum; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Laurentin, H.; Ratzinger, A.; Karlovsky, P. Relationship between metabolic and genomic diversity in sesame (Sesamum indicum L.). BMC Genomics 2008, 9, 250. [Google Scholar] [CrossRef] [Green Version]
- Mochida, K.; Furuta, T.; Ebana, K.; Shinozaki, K.; Kikuchi, J. Correlation exploration of metabolic and genomic diversity in rice. BMC Genom. 2009, 10, 568. [Google Scholar] [CrossRef] [Green Version]
- Houshyani, B.; Kabouw, P.; Muth, D.; de Vos, R.C.H.; Bino, R.J.; Bouwmeester, H.J. Characterization of the natural variation in Arabidopsis thaliana metabolome by the analysis of metabolic distance. Metabolomics 2012, 8, 131–145. [Google Scholar] [CrossRef] [Green Version]
- Sarrou, E.; Ganopoulos, I.; Xanthopoulou, A.; Masuero, D.; Martens, S.; Madesis, P.; Mavromatis, A.; Chatzopoulou, P. Genetic diversity and metabolic profile of Salvia officinalis populations: Implications for advanced breeding strategies. Planta 2017, 246, 201–215. [Google Scholar] [CrossRef]
- Watanabe, S.; Ohtani, Y.; Aoki, W.; Uno, Y.; Sukekiyo, Y.; Kubokawa, S.; Ueda, M. Detection of betacyanin in red-tube spinach (Spinacia oleracea) and its biofortification by strategic hydroponics. PLoS ONE 2018, 13, e0203656. [Google Scholar] [CrossRef] [Green Version]
- Laurentin, H.; Karlovsky, P. AFLP fingerprinting of sesame (Sesamum indicum L.) cultivars: Identification, genetic relationship and comparison of AFLP informativeness parameters. Genet. Resour. Crop Evol. 2007, 54, 1437–1446. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, L.; Miao, H.; Zhang, T.; Wang, C. Development and validation of genic-SSR markers in sesame by RNA-seq. BMC Genom. 2012, 13, 316. [Google Scholar] [CrossRef] [Green Version]
- Kareem, Z.J.; Su, L.; Rathgeb, A.; Sirrenberg, A.; Hadacek, F.; Rashid, A.H.A.; Karlovsky, P. Small-scale bioreactor for sterile hydroponics and hairy roots: Metabolic diversity and salicylic acid exudation by hairy roots of Hyoscyamus niger. Appl. Sci. 2019, 9, 3044. [Google Scholar] [CrossRef] [Green Version]
- Khanna, P.; Jain, S.C. Isolation and identification of sesamin from Sesamum indicum tissue culture. Curr. Sci. 1973, 42, 253–254. [Google Scholar]
- Mimura, A.; Takebayashi, K.; Niwano, M.; Takahara, Y.; Osawa, T.; Tokuda, H. Antioxidative and anticancer components produced by cell culture of sesame. In Food Phytochemicals for Cancer Prevention II; Ho, C.T., Osawa, T., Huang, M.T., Rosen, R.T., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1994; pp. 281–294. [Google Scholar]
- Ogasawara, T.; Chiba, K.; Tada, M. XIX Sesamum indicum L. (Sesame): In vitro culture, and the production of naphthoquinone and other secondary metabolites. In Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1998; Volume 41, pp. 366–393. [Google Scholar]
- Mimura, A.; Ichikawa, A.; Takahara, Y.; Osawa, T. Production of antioxidants by tissue culture of Sesamum indicum L. Nippon Nogeikagaku Kaishi 1987, 61, 479. (In Japanese) [Google Scholar]
- Mimura, A.; Takahara, Y.; Ichikawa, A.; Osawa, T. The manufacture of plant tissue culture containing antioxidative compounds. Jpn. Kokai Tokkyo Koho 1987, 87, 40094. (In Japanese) [Google Scholar]
- Mimura, A.; Takahara, Y.; Ichikawa, A.; Osawa, T. Lignan compounds and their manufacture with tissue cultrue of Sesamum indicum. Jpn. Kokai Tokkyo Koho 1987, 87, 40096. (In Japanese) [Google Scholar]
- Takebayashi, K.; Mimura, A.; Ichikawa, A.; Niwano, M.; Takahara, Y.; Osawa, T. Cultivation of Sesamum indicum L. callus cells at 35 °C. Plant Tissue Cult. Lett. 1994, 11, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Kareem, Z.J. Biomedical Applications and Secondary Metabolite Profiling of Hyoscyamus niger and Sesamum indicum Seed, Root and Hairy Root Cultures. Ph.D. Thesis, University of Goettingen, Goettingen, Germany, 2020. [Google Scholar]
- Hata, N.; Hayashi, Y.; Okazawa, A.; Ono, E.; Satake, H.; Kobayashi, A. Comparison of sesamin contents and CYP81Q1 gene expressions in above ground vegetative organs between two japanese sesame (Sesamum indicum L.) varieties differing in seed sesamin contents. Plant Sci. 2010, 178, 510–516. [Google Scholar] [CrossRef]
- Fuji, Y.; Ohtsuki, T.; Matsufuji, H. Accumulation and subcellular localization of acteoside in sesame plants (Sesamum indicum L.). ACS Omega 2018, 3, 17287–17294. [Google Scholar] [CrossRef] [Green Version]
- Pearman, R.W.; Raymond, W.D.; Squires, J.A. Wild sesame from northern Rhodesia. Colonial Plant Animal Prod. 1951, 2, 297–299. [Google Scholar]
- Hwang, L.S. Sesame Oil. In Bailey’s Industrial Oil and Fat Products. Vol. 2 Edible Oil and Fat Products; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Davin, L.B.; Lewis, N.G. An historical perspective on lignan biosynthesis: Monolignol, allylphenol and hydroxycinnamic acid coupling and downstream metabolism. Phytochem. Rev. 2003, 2, 257. [Google Scholar] [CrossRef]
- Suzuki, S.; Umezawa, T. Biosynthesis of lignans and norlignans. J. Wood Sci. 2007, 53, 273–284. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.W.; Smith, C.A.; Daily, M.D.; Cort, J.R.; Davin, L.B.; Norman, G.L. Trimeric structure of (+)-pinoresinol-forming dirigent protein at 1.95 Å resolution with three isolated active sites. J. Biol. Chem. 2015, 290, 1308–1318. [Google Scholar] [CrossRef] [Green Version]
- Davin, L.B.; Wang, H.B.; Crowell, A.L.; Bedgar, D.L.; Martin, D.M.; Sarkanen, S.; Lewis, N.G. Stereoselective biomolecu-lar phenoxy radical coupling by an auxiliary (dirigent) protein without an active center. Science 1997, 275, 362–366. [Google Scholar] [CrossRef]
- Davin, L.B.; Lewis, N.G. Dirigent proteins and dirigent sites explain the mystery of specificity of radical precursor coupling in lignan and lignin biosynthesis. Plant Physiol. 2000, 123, 453–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Davin, L.B.; Lewis, N.G. Furofuran lignan metabolism as a function of seed maturation in Sesamum indicum: Methylenedioxy bridge formation. Phytochemistry 1998, 49, 387–394. [Google Scholar] [CrossRef]
- Chandra, K.; Sinha, A.; Arumugam, N. Gene isolation, heterologous expression, purification and functional confirmation of sesamin synthase from Sesamum indicum L. Biotechnol. Rep. 2019, 22, e00336. [Google Scholar] [CrossRef]
- Xia, Z.Q.; Costa, M.A.; Péllisier, H.; Davin, L.B.; Lewis, N.G. Secoisolariciresinol dehydrogenase purification, cloning and functional expression. J. Biol. Chem. 2001, 276, 12614–12623. [Google Scholar] [CrossRef] [Green Version]
- Markulin, L.; Corbin, C.; Renouard, S.; Drouet, S.; Gutierrez, L.; Mateljak, I.; Auguin, D.; Hano, C.; Fuss, E.; Lainé, E. Pinoresinol–lariciresinol reductases, key to the lignan synthesis in plants. Planta 2019, 249, 1695–1714. [Google Scholar] [CrossRef]
- Murata, J.; Ono, E.; Yoroizuka, S.; Toyonaga, H.; Shiraishi, A.; Mori, S.; Tera, M.; Azuma, T.; Nagano, A.J.; Nakayasu, M.; et al. Oxidative rearrangement of (+)-sesamin by CYP92B14 co-generates twin dietary lignans in sesame. Nat. Commun. 2017, 8, 2155. [Google Scholar] [CrossRef]
- Noguchi, A.; Fukui, Y.; Iuchi-Okada, A.; Kakutani, S.; Satake, H.; Iwashita, T.; Nakao, M.; Umezawa, T.; Ono, E. Sequential glucosylation of a furofuran lignan, (+)-sesaminol, by Sesamum indicum UGT71A9 and UGT94D1 glucosyltransferases. Plant J. 2008, 54, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Waki, T.; Oikawa, D.; Murata, J.; Shiraishi, A.; Toyonaga, H.; Kato, M.; Ogata, N.; Takahashi, S.; Yamaguchi, M.; et al. Glycoside-specific glycosyltransferases catalyze regioselective sequential glucosylations for a sesame lignan, sesaminol triglucoside. Plant J. 2020, 101, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.V.; Kim, K.-W.; Lee, C.; Costa, M.A.; May, G.D.; Crow, J.A.; Davin, L.B.; Lewis, N.G. Next generation sequencing in predicting gene function in podophyllotoxin biosynthesis. J. Biol. Chem. 2013, 288, 466–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umezawa, T. Diversity in lignan biosynthesis. Phytochem. Rev. 2003, 2, 371–390. [Google Scholar] [CrossRef]
- Umezawa, T.; Shimada, M. Formation of the lignan (+)-Secoisolariciresinol by cell-free extracts of Arctium lappa. Biosci. Biotechnol. Biochem. 1996, 60, 736–737. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.D.A.; Katayama, T.; Suzuki, T.; Nakagawa, T. Stereochemistry and biosynthesis of (+)-lyoniresinol, a syringyl tetrahydronaphthalene lignan in Lyonia ovalifolia var. elliptica I: Isolation and stereochemistry of syringyl lignans and predicted precursors to (+)-lyoniresinol from wood. J. Wood Sci. 2007, 53, 161–167. [Google Scholar] [CrossRef]
- Kato, M.J.; Chu, A.; Davin, L.B.; Lewis, N.G. Biosynthesis of antioxidant lignans in Sesamum indicum seeds. Phytochemistry 1998, 47, 583–591. [Google Scholar] [CrossRef]
- Broomhead, A.J.; Dewick, P.M. Aryltetralin lignans from Linum flavum and Linum capitatum. Phytochemistry 1990, 29, 3839–3844. [Google Scholar] [CrossRef]
- Okunishi, T.; Umezawa, T.; Shimada, M. Enantiomeric compositions and biosynthesis of Wikstroemia sikokiana lignans. J. Wood Sci. 2000, 46, 234–242. [Google Scholar] [CrossRef]
- Rahman, M.M.A.; Dewick, P.M.; Jackson, D.E.; Lucas, J.A. Biosynthesis of lignans in Forsythia intermedia. Phytochemistry 1990, 29, 1841–1846. [Google Scholar] [CrossRef]
- Umezawa, T.; Davin, L.B.; Yamamoto, E.; Kingston, D.G.I.; Lewis, N.G. Lignan biosynthesis in forsythia species. J. Chem. Soc. Chem. Commun. 1990, 1405–1408. [Google Scholar] [CrossRef]
- Katayama, T.; Davin, L.B.; Chu, A.; Lewis, N.G. Novel benzylic ether reductions in lignan biogenesis in Forsythia intermedia. Phytochemistry 1993, 33, 581–591. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Gang, D.R.; Davin, L.B.; Bedgar, D.L.; Chu, A.; Lewis, N.G. (+)-Pinoresinol/(+)-lariciresinol reductase from Forsythia intermedia. J. Biol. Chem. 1996, 271, 29473–29482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidel, V.; Windhövel, J.; Eaton, G.; Alfermann, W.A.; Arroo, R.R.; Medarde, M.; Petersen, M.; Woolley, J.G. Biosynthesis of podophyllotoxin in Linum album cell cultures. Planta 2002, 215, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, S.; Kranz, K.; Lücking, B.; Alfermann, A.W.; Petersen, M. Aspects of cytotoxic lignan biosynthesis in suspension cultures of Linum nodiflorum. Phytochem. Rev. 2002, 1, 37–43. [Google Scholar] [CrossRef]
- Federolf, K.; Alfermann, A.W.; Fuss, E. Aryltetralin-lignan formation in two different cell suspension cultures of Linum album: Deoxypodophyllotoxin 6-hydroxylase, a key enzyme for the formation of 6-methoxypodophyllotoxin. Phytochemistry 2007, 68, 1397–1406. [Google Scholar] [CrossRef]
- Ozawa, S.; Davin, L.B.; Lewis, N.G. Formation of (−)-arctigenin in Forsythia intermedia. Phytochemistry 1993, 32, 643–652. [Google Scholar] [CrossRef]
- Shiraishi, A.; Murata, J.; Matsumoto, E.; Matsubara, S.; Ono, E.; Satake, H. De novo transcriptomes of Forsythia koreana using a novel assembly method: Insight into tissue- and species-specific expression of lignan biosynthesis-related gene. PLoS ONE 2016, 11, e0164805. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.; Sattely, E.S. Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 2015, 349, 1224–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molog, G.; Empt, U.; Kuhlmann, S.; van Uden, W.; Pras, N.; Alfermann, A.; Petersen, M. Deoxypodophyllotoxin 6-hydroxylase, a cytochrome P450 monooxygenase from cell cultures of Linum flavum involved in the biosynthesis of cytotoxic lignans. Planta 2001, 214, 288–294. [Google Scholar] [CrossRef]
- Ono, E.; Kim, H.J.; Murata, J.; Morimoto, K.; Okazawa, A.; Kobayashi, A.; Umezawa, T.; Satake, H. Molecular and functional characterization of novel furofuran-class lignan glucosyltransferases from Forsythia. Plant Biotechnol. 2010, 27, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Ghose, K.; Selvaraj, K.; McCallum, J.; Kirby, C.W.; Sweeney-Nixon, M.; Cloutier, S.J.; Deyholos, M.; Datla, R.; Fofana, B. Identification and functional characterization of a flax UDP-glycosyltransferase glucosylating secoisolariciresinol (SECO) into secoisolariciresinol monoglucoside (SMG) and diglucoside (SDG). BMC Plant Biol. 2014, 14, 82. [Google Scholar] [CrossRef] [Green Version]
- Kliebenstein, D.J.; Osbourn, A. Making new molecules evolution of pathways for novel metabolites in plants. Curr. Opin. Plant Biol. 2012, 15, 415–423. [Google Scholar] [CrossRef]
- Guo, N.; Cheng, F.; Wu, J.; Liu, B.; Zheng, S.; Liang, J.; Wang, X. Anthocyanin biosynthetic genes in Brassica rapa. BMC Genomics 2014, 15, 426. [Google Scholar] [CrossRef] [Green Version]
- Nützmann, H.W.; Huang, A.; Osbourn, A. Plant metabolic clusters from genetics to genomics. New Phytologist 2016, 211, 771–789. [Google Scholar] [CrossRef] [Green Version]
- Wright, F. The effective number of codons used in a gene. Gene 1990, 87, 23–29. [Google Scholar] [CrossRef]
- Tera, M.; Koyama, T.; Murata, J.; Furukawa, A.; Mori, S.; Azuma, T.; Watanabe, T.; Hori, K.; Okazawa, A.; Kabe, Y.; et al. Identification of a binding protein for sesamin and characterization of its roles in plant growth. Sci. Rep. 2019, 9, 8631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hata, N.; Hayashi, Y.; Okazawa, A.; Ono, E.; Satake, H.; Kobayashi, A. Effect of photoperiod on growth of the plants, and sesamin content and CYP81Q1 gene expression in the leaves of sesame (Sesamum indicum L.). Environ. Exp. Bot. 2012, 75, 212–219. [Google Scholar] [CrossRef]
- Zhong, R.; Richardson, E.A.; Ye, Z.-H. Two NAC domain transcription factors, SND1 and NST1, function redundantly in regulation of secondary wall synthesis in fibers of Arabidopsis. Planta 2007, 225, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M. Identification of Genes Controlling the Contents of Sesame Seed Lignans; Project Report; University of Toyama: Toyama, Japan, 2015. (In Japanese) [Google Scholar]
- Kato, M.; Ogata, N.; Katsuta, M.; Yamada, T.; Sugiura, M.; Yasumotoa, S. Breeding of the high-lignan sesame variety Nishikimaru. Bull. NARO Crop Sci. 2017, 1, 125–143. (In Japanese) [Google Scholar]
- Kamal-Eldin, A.; Moazzami, A.; Waxhi, S. Sesame seed lignans: Potent physiological modulators and possible ingredients in functional foods & nutraceuticals. Recent Pat. Food Nutr. Agric. 2011, 3, 17–19. [Google Scholar]
- Bruce, R.A.; Tobin, C.E. The effects of sesame oil and fractions of sesame oil on adrenalectomized and other experimental rats. Endocrinology 1940, 27, 956–970. [Google Scholar] [CrossRef]
- Tobin, C.E. The effect of adrenalectomy on pregnancy and survival of untreated and sesame oil treated rats. Endocrinology 1941, 28, 419–425. [Google Scholar] [CrossRef]
- Pilkington, L.I. Lignans: A chemometric analysis. Molecules 2018, 23, 1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, R.S.; Katyal, A. Myeloperoxidase: Bridging the gap in neurodegeneration. Neurosci. Biobehav. Rev. 2016, 68, 611–620. [Google Scholar] [CrossRef]
- Andraos, V.; Swift, C.E.; Dollear, F.G. Sesame oil. I. Properties of a solvent-extracted sesame oil. J. Am. Oil Chem. Soc. 1950, 27, 31–34. [Google Scholar] [CrossRef]
- Wan, Y.; Li, H.; Fu, G.; Chen, X.; Chen, F.; Xie, M. The relationship of antioxidant components and antioxidant activity of sesame seed oil. J. Sci. Food Agric. 2015, 95, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Zheng, G.H.; Ming, Q.L.; Chao, C.; Sun, J.M. Sesamin protects mouse liver against nickel-induced oxidative DNA damage and apoptosis by the PI3K-Akt pathway. J. Agric. Food Chem. 2013, 61, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.H.; Mait, M.; Tsujihara, N.; Osawa, T. Sesamolin inhibits lipid peroxidation in rat liver and kidney. J. Nutr. 1998, 128, 1018–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suja, K.P.; Jayalekshmy, A.; Arumughan, C. In Vitro studies on antioxidant activity of lignans isolated from sesame cake extract. J. Sci. Food Agric. 2005, 85, 1779–1783. [Google Scholar] [CrossRef]
- Steffan, B.; Wätjen, W.; Michels, G.; Niering, P.; Wray, V.; Ebel, R.; Edrada, R.; Kahl, R.; Proksch, P. Polyphenols from plants used in traditional Indonesian medicine (Jamu): Uptake and antioxidative effects in rat H4IIE hepatoma cells. J. Pharm. Pharmacol. 2005, 57, 233–240. [Google Scholar] [CrossRef]
- López-Biedma, A.; Sánchez-Quesada, C.; Beltrán, G.; Delgado, M.; Gaforio, J.J. Phytoestrogen (+)-pinoresinol exerts antitumor activity in breast cancer cells with different oestrogen receptor statuses. BMC Complement. Altern. Med. 2016, 16, 350. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.H.; Kawai, Y.; Naito, M.; Osawa, T. Dietary defatted sesame flour decreases susceptibility to oxidative stress in hypercholesterolemic rabbits. J. Nutr. 1999, 129, 1885–1890. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Choe, E.O. Extraction of lignan compounds from roasted sesame oil and their effects on the autoxidation of methyl linoleate. J. Food Sci. 2006, 71, C430–C436. [Google Scholar] [CrossRef]
- Kumagai, Y.; Lin, L.Y.; Schmitz, D.A.; Cho, A.K. Hydroxyl radical mediated demethylenation of (methlenedioxyl) phenyl compounds. Chem. Res. Toxicol. 1991, 4, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Osawa, T.; Ide, A.; Jeng, D.S.; Namiki, M. Inhibition of lipid peroxidation by ellagic acid. J. Agric. Food Chem. 1987, 35, 808–812. [Google Scholar] [CrossRef]
- Hiramoto, K.; Ojima, N.; Sako, K.; Kikugawa, K. Effect of plant phenolics on the formation of the spin-adduct of hydroxyl radical and the DNA strand breaking by hydroxyl radical. Biol. Pharm. Bull. 1996, 19, 558–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joshi, R.; Kumar, M.S.; Satyamoorthy, K.; Unnikrishnan, M.K.; Mukherjee, T. Free radical reactions and antioxidant activities of sesamol: Pulse radiolytic and biochemical studies. J. Agric. Food Chem. 2005, 53, 2696–2703. [Google Scholar] [CrossRef]
- Kaur, I.P.; Saini, A. Sesamol exhibits antimutagenic activity against oxygen species mediated mutagenicity. Mutat. Res. 2000, 10, 71–76. [Google Scholar] [CrossRef]
- Wu, J.H.; Hodgson, J.M.; Clarke, M.W.; Indrawan, A.P.; Barden, A.E.; Puddey, I.B.; Croft, K.D. Inhibition of 20-hydroxyeicosatetraenoic acid synthesis using specific plant lignans: In vitro and human studies. Hypertension 2009, 54, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Hanzawa, F.; Nomura, S.; Sakuma, E.; Uchida, T.; Ikeda, S. Dietary sesame seed and its lignan, sesamin, increase tocopherol and phylloquinone concentrations in male rats. J. Nutr. 2013, 143, 1067–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, R.S.; Sontag, T.J.; Swanson, J.E. Cytochrome P4503A-dependent metabolism of tocopherols and inhibition by sesamin. Biochem. Biophys. Res. Commun. 2000, 277, 531–534. [Google Scholar] [CrossRef]
- Moutsatsou, P. The spectrum of phytoestrogens in nature: Our knowledge is expanding. Hormones 2007, 6, 173–193. [Google Scholar]
- Mostrom, M.; Evans, T.J. Phytoestrogens. In Veterinary Toxicology-Basic and Clinical Principles, 2nd ed.; Gupta, R.C., Ed.; Academic Press: London, UK, 2012; pp. 1012–1028. [Google Scholar]
- Wu, W.H.; Kang, Y.P.; Wang, N.H.; Jou, H.J.; Wang, T. Sesame ingestion affects sex hormones, antioxidant status, and blood lipids in postmenopausal women. J. Nutr. 2006, 136, 1270–1275. [Google Scholar] [CrossRef]
- Kaur, A.; Jindal, S.; Kaur, I.P.; Chopra, K. Effect of sesamol on the pathophysiological changes induced by surgical menopause in rodents. Climacteric 2013, 16, 426–437. [Google Scholar] [CrossRef]
- Bedell, S.; Nachtigal, M.; Naftolin, F. The pros and cons of plant estrogens for menopause. J. Steroid Biochem. Mol. Biol. 2014, 139, 225–236. [Google Scholar] [CrossRef]
- Unkila, M. The Use of a Lignan for the Manufacture of a Composition for Preventing or Alleviating of Symptoms Relating to Estrogen Deficiency. U.S. Patent Application No 11/813,589, 6 March 2008. [Google Scholar]
- Zhu, Y.; Kawaguchi, K.; Kiyama, R. Differential and directional estrogenic signaling pathways induced by enterolignans and their precursors. PLoS ONE 2017, 12, e0171390. [Google Scholar] [CrossRef] [Green Version]
- Pianjing, P.; Thiantanawat, A.; Rangkadilok, N.; Watcharasit, P.; Mahidol, C.; Satayavivad, J. Estrogenic activities of sesame lignans and their metabolites on human breast cancer cells. J. Agric. Food Chem. 2011, 59, 212–221. [Google Scholar] [CrossRef]
- Shittu, L.A.J.; Shittu, R.K.; Samson, A.O.; Ajala, M.O.; Munir, B.A.; Benebo, A.S.; Tayo, A.O.; Olufemi, O.A.; Oladapo, A.A. Sesamum radiatum phytoestrogens stimulate spermatogenic activity and improve sperm quality in adult male sprague dawley rat testis. Int. J. Morphol. 2008, 26, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Aehle, E.; Müller, U.; Eklund, P.C.; Willför, S.M.; Sippl, W.; Dräger, B. Lignans as food constituents with estrogen and antiestrogen activity. Phytochemistry 2011, 72, 2396–2405. [Google Scholar] [CrossRef]
- Kato, T.; Harashima, T.; Moriya, N.; Kikugawa, K.; Hiramoto, K. Formation of the mutagenic/carcinogenic imidazoquinoxaline-type heterocyclic amines through the unstable free radical Maillard intermediates and its inhibition by phenolic antioxidants. Carcinogenesis 1996, 17, 2469–2476. [Google Scholar] [CrossRef] [Green Version]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Massri, M.; Nasrallah, G.K. A comprehensive review on the anti-cancer properties and mechanisms of action of sesamin, a lignan in sesame seeds (Sesamum indicum). Eur. J. Pharmacol. 2017, 815, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Mali, A.V.; Padhye, S.B.; Anant, S.; Hegde, M.V.; Kadam, S.S. anticancer and antimetastatic potential of enterolactone: Clinical, preclinical and mechanistic perspectives. Eur. J. Pharmacol. 2019, 852, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Hirose, N.; Doi, F.; Ueki, T.; Akazawa, K.; Chijiiwa, K.; Sugano, M.; Akimoto, K.; Shimizu, S.; Yamada, H. Suppressive effect of sesamin against 7,12-dimethylbenzanthracene induced rat mammary carcinogenesis. Anticancer Res. 1992, 12, 1259–1266. [Google Scholar] [PubMed]
- Chen, J.-M.; Chen, P.-Y.; Lin, C.-C.; Hsieh, M.-C.; Lin, J.-T. Antimetastatic effects of sesamin on human head and neck squamous cell carcinoma through regulation of matrix metalloproteinase-2. Molecules 2020, 25, 2248. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, G.J.; Azuine, M.A.; Tokuda, H.; Takasaki, M.; Mukainaka, T. Chemopreventive effect of resveratrol, sesamol, sesame oil and sunflower oil in the Epstein-Barr virus early antigen activation assay and the mouse skin two-stage carcinogenesis. Pharmacol. Res. 2002, 45, 499–505. [Google Scholar] [CrossRef]
- Siriwarin, B.; Weerapreeyakul, N. Sesamol induced apoptotic effect in lung adenocarcinoma cells through both intrinsic and extrinsic pathways. Chem. Biol. Interact. 2016, 254, 109–116. [Google Scholar] [CrossRef]
- Miyahara, Y.; Hibasami, H.; Katsuzaki, H.; Imai, K.; Osawa, T.; Ina, K.; Komiya, T. Sesaminol from sesame seed induces apoptosis in human lymphoid leukemia Molt 4B cells. Inter. J. Mol. Med. 2001, 7, 485–488. [Google Scholar] [CrossRef]
- Ryu, S.N.; Kim, K.S.; Kang, S.S. Growth inhibitory effects of sesamolin from sesame seeds on human leukemia HL-60 cells. Korean J. Pharm. 2003, 34, 237–241. [Google Scholar]
- Sheng, H.Q.; Hirose, Y.; Hata, K.; Zheng, Q.; Kuno, T.; Asano, N.; Yamada, Y.; Hara, A.; Osawa, T.; Mori, H. Modifying effect of dietary sesaminol glucosides on the formation of azoxymethane induced premalignant lesions of rat colon. Cancer Lett. 2007, 246, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Iizumi, Y.; Sukeno, M.; Iizuka-Ohashi, M.; Sowa, Y.; Sakai, T. The pleiotropic regulation of cyclin D1 by newly identified sesaminol-binding protein ANT2. Oncogenesis 2017, 6, e311. [Google Scholar] [CrossRef] [Green Version]
- Shimoyoshi, S.; Takemoto, D.; Ono, Y.; Kitagawa, Y.; Shibata, H.; Tomono, S.; Unno, K.; Wakabayashi, K. Sesame lignans suppress age-related cognitive decline in senescence-accelerated mice. Nutrients 2019, 11, 1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, A.; Oda, Y.; Ito, N.; Seki, S.; Nakagawa, K.; Miyazawa, T.; Ueda, F. Eeffects of dietary supplementation of astaxanthin and sesamin on daily fatigue: A randomized, double-blind, placebo-controlled, two-way crossover study. Nutrients 2018, 10, 281. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.; ElSherbiny, N.M.; Haque, R.; Khan, M.B.; Ishrat, T.; Shah, Z.A.; Khan, M.M.; Ali, M.; Jamal, A.; Katare, D.P.; et al. Sesamin attenuates neurotoxicity in mouse model of ischemic brain stroke. Neurotoxicology 2014, 45, 100–110. [Google Scholar] [CrossRef]
- Khan, M.M.; Ishrat, T.; Ahmad, A.; Hoda, M.N.; Khan, M.B.; Khuwaja, G.; Srivastava, P.; Raza, S.S.; Islam, F.; Ahmad, S. Sesamin attenuates behavioral, biochemical and histological alterations induced by reversible middle cerebral artery occlusion in the rats. Chem. Biol. Interact. 2010, 183, 255–263. [Google Scholar] [CrossRef]
- Cheng, F.C.; Jinn, T.R.; Hou, R.C.; Tzen, J.T. Neuroprotective effects of sesamin and sesamolin on gerbil brain in cerebral ischemia. Int. J. Biomed. Sci. 2006, 2, 284–288. [Google Scholar] [PubMed]
- Jayaraj, P.; Narasimhulu, C.A.; Rajagopalan, S.; Parthasarathy, S.; Desikan, R. Sesamol: A powerful functional food ingredient from sesame oil for cardioprotection. Food Funct. 2020, 11, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Kushiro, M.; Takahashi, Y.; Shinohara, K.; Fukuda, N.; Sirato-Yasumoto, S. Sesamin, a sesame lignan, as a potent serum lipid lowering food component. Japan Agric. Res. Quart. 2003, 37, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Akimoto, K.; Kitagawa, Y.; Akamatsu, T.; Hirose, M.; Sugano, M.; Shimizu, S.; Yamada, H. Protective effects of sesamin against liver damage caused by alcohol or carbon tetrachloride in rodents. Ann. Nutr. Metab. 1993, 37, 218–224. [Google Scholar] [CrossRef]
- Hirose, N.; Inoue, T.; Nishihara, K.; Sugano, M.; Akimoto, K.; Shimizu, S.; Yamada, H. Inhibition of cholesterol absorption and synthesis in rats by sesamin. J. Lipid Res. 1991, 32, 629–638. [Google Scholar] [CrossRef]
- Penalvo, J.L.; Hopia, A.; Adlercreutz, H. Effect of sesamin on serum cholestrol and triglycerides level in LDL-receptor-deficient mice. Eur. J. Nutr. 2006, 45, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, T.; Ikeda, K.; Sasaki, Y.; Yamamoto, J.; Seki, J.; Yamagata, K.; Nara, Y.; Hara, H.; Kakuta, H.; Yamori, Y. Effects of vitamin E and sesamin on hypertension and cerebral thrombogenesis in stroke-prone spontaneously hypertensive rats. Hypertens Res 2001, 24, 735–742. [Google Scholar] [CrossRef] [Green Version]
- Thuy, T.D.; Phan, N.N.; Wang, C.Y.; Yu, H.G.; Wang, S.Y.; Huang, P.L.; Do, Y.Y.; Lin, Y.C. Novel therapeutic effects of sesamin on diabetes-induced cardiac dysfunction. Mol. Med. Rep. 2017, 15, 2949–2956. [Google Scholar] [CrossRef]
- Miyawaki, T.; Aono, H.; Toyoda-Ono, Y.; Maeda, H.; Kiso, Y.; Moriyama, K. Antihypertensive effects of sesamin in humans. J. Nutr. Sci. Vitaminol. 2009, 55, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Hirata, F.; Fujita, K.; Ishikura, Y.; Hosoda, K.; Ishikawa, T.; Nakamura, H. Hypocholesterolemic effect of sesame lignan in humans. Atherosclerosis 1996, 122, 135–136. [Google Scholar] [CrossRef]
- Ndrepepa, G. Myeloperoxidase—A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta 2019, 493, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Jin, S.W.; Lee, G.H.; Park, J.S.; Kim, J.Y.; Thai, T.N.; Han, E.H.; Jeong, H.G. Sesamin induces endothelial nitric oxide synthase activation via transient receptor potential vanilloid type 1. J. Agric. Food Chem. 2020, 68, 3474–3484. [Google Scholar] [CrossRef] [PubMed]
- Comhaire, F.; Mahmoud, A. Anti-Ageing Nutrition and Food Supplements. In Andrology for the Clinician; Schill, W.B., Comhaire, F., Hargreave, T.B., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 565–571. [Google Scholar]
- Anonymous. Lignans Market Size, Share & Trends Analysis Report; Grand View Research: San Francisco, CA, USA, 2020; pp. 1–145. [Google Scholar]
- Zuo, Y.; Peng, C.; Liang, Y.; Ma, K.Y.; Chan, H.Y.E.; Huang, Y.; Chen, Z.-Y. Sesamin extends the mean lifespan of fruit flies. Biogerontology 2013, 14, 107–119. [Google Scholar] [CrossRef]
- Yaguchi, Y.; Komura, T.; Kashima, N.; Tamura, M.; Kage-Nakadai, E.; Saeki, S.; Terao, K.; Nishikawa, Y. Influence of oral supplementation with sesamin on longevity of Caenorhabditis elegans and the host defense. Eur. J. Nutr. 2014, 53, 1659–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakatani, Y.; Yaguchi, Y.; Komura, T.; Nakadai, M.; Terao, K.; Kage-Nakadai, E.; Nishikawa, Y. Sesamin extends lifespan through pathways related to dietary restriction in Caenorhabditis elegans. Eur. J. Nutr. 2018, 57, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Le, T.D.; Nakahara, Y.; Ueda, M.; Okumura, K.; Hirai, J.; Sato, Y.; Takemoto, D.; Tomimori, N.; Ono, Y.; Nakai, M.; et al. Sesamin suppresses aging phenotypes in adult muscular and nervous systems and intestines in a Drosophila senescence-accelerated model. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1826–1839. [Google Scholar]
- Lei, H.; Cai, L.; Wang, Y.; Han, J.; Wang, Q.; Zhang, X. Anti-aging effect of sesamin and its mechanism of action. Curr. Top. Nutraceut. Res. 2012, 10, 173. [Google Scholar]
- Shimoyoshi, S.; Takemoto, D.; Kishimoto, Y.; Amano, A.; Sato, A.; Ono, Y.; Rogi, T.; Shibata, H.; Ishigami, A. Sesame lignans suppress age-related disorders of the kidney in mice. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5140–5147. [Google Scholar]
- Sharma, S.; Kaur, I.P. Development and evaluation of sesamol as an antiaging agent. Int. J. Dermatol. 2006, 45, 200–208. [Google Scholar] [CrossRef]
- Loy, C.J.; Mahmook, K.; Saliou, C. Compositions Comprising Paulownin and/or Paulownia Extracts and Uses Thereof. U.S. Patent No. 9,168,279, 17 October 2015. [Google Scholar]
- Kaur, S.; Loy, C.J.; Mahmood, K. Compositions Comprising Paulownin and/or Paulownia Extracts and Uses Thereof. EPO Patent Application No. 2 605 749, 22 November 2017. [Google Scholar]
- Kiran, K.; Asad, M. Wound healing activity of Sesamum indicum L. seed and oil in rats. Indian J. Exp. Biol. 2008, 46, 777–782. [Google Scholar]
- Shenoy, R.R.; Sudheendra, A.T.; Nayak, P.G.; Paul, P.; Kutty, N.G.; Rao, C.M. Normal and delayed wound healing is improved by sesamol, an active constituent of Sesamum indicum (L.) in albino rats. J. Ethnopharmacol. 2011, 133, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, X.; Wang, L.; Yan, X.; Ma, D.; Liu, Z.; Liu, X. Sesamol incorporated cellulose acetate-zein composite nanofiber membrane: An efficient strategy to accelerate diabetic wound healing. Int. J. Biol. Macromol. 2020, 149, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Jeng, K.C.; Hou, R.C. Sesamin and sesamolin: Natures therapeutic lignans. Curr. Enz. Inhibit. 2005, 1, 11–20. [Google Scholar] [CrossRef]
- Monteiro, E.M.H.; Chibli, L.A.; Yamamoto, C.H.; Pereira, M.C.S.; Vilela, F.M.P.; Rodarte, M.P.; Pinto, M.A.D.O.; do Amaral, M.D.P.H.; Silvério, M.S.; Araújo, A.L.S.D.M.; et al. Antinociceptive and anti-inflammatory activities of sesame oil and sesamin. Nutrients 2014, 6, 1931–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, S.; El Sherbiny, N.M.; Jamal, M.S.; Alzahrani, F.A.; Haque, R.; Khan, R.; Zaidi, S.K.; Al Qahtani, M.H.; Liou, G.I.; Bhatia, K. Anti-inflammatory role of sesamin in STZ induced mice model of diabetic retinopathy. J. Neuroimmunol. 2016, 295–296, 47–53. [Google Scholar] [CrossRef]
- Ma, J.Q.; Ding, J.; Zhang, L.; Liu, C.M. Hepatoprotective properties of sesamin against CCl4 induced oxidative stress-mediated apoptosis in mice via JNK pathway. Food Chem. Toxicol. 2014, 64, 41–48. [Google Scholar] [CrossRef]
- Eweda, S.M.; Newairy, A.S.A.; Abdou, H.M.; Gaber, A.S. Bisphenol A-induced oxidative damage in the hepatic and cardiac tissues of rats: The modulatory role of sesame lignans. Exp. Theor. Med. 2020, 19, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Kwon, D.; Kim, G.D.; Kang, W.; Park, J.-E.; Kim, S.H.; Choe, E.; Kim, J.-I.; Auh, J.-H. Pinoresinol diglucoside is screened as a putative α-glucosidase inhibiting compound in Actinidia arguta leaves. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 473–479. [Google Scholar] [CrossRef]
- Worawalai, W.; Doungwichitrkul, T.; Rangubpit, W.; Taweechat, P.; Sompornpisut, P.; Phuwapraisirisan, P. furofuran lignans as a new series of antidiabetic agents exerting α-glucosidase inhibition and radical scarvenging: Semisynthesis, kinetic study and molecular modeling. Bioorg. Chem. 2019, 87, 783–793. [Google Scholar] [CrossRef]
- Phitak, T.; Pothacharoen, P.; Settakorn, J.; Poompimol, W.; Caterson, B.; Kongtawelert, P. Chondroprotective and anti-inflammatory effects of sesamin. Phytochemistry 2012, 80, 77–88. [Google Scholar] [CrossRef]
- Wu, M.S.; Aquino, L.B.B.; Barbaza, M.Y.U.; Hsieh, C.L.; De Castro-Cruz, K.A.; Yang, L.L.; Tsai, P.W. Anti-inflammatory and anticancer properties of bioactive compounds from Sesamum indicum L.—A review. Molecules 2019, 24, 4426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamano, S.; Hirose, M.; Tanaka, H.; Asakawa, E.; Ogawa, K.; Ito, N. Forestomach neoplasm induction in F344/DuCrj rats and B6C3F1 mice exposed to sesamol. Jpn. J. Cancer Res. 1992, 83, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Geetha, T.; Singh, N.; Deol, P.K.; Kaur, I.P. Biopharmaceutical profiling of sesamol: Physiochemical characterization, gastrointestinal permeability and pharmacokinetic evaluation. RSC Adv. 2014, 5, 4083–4091. [Google Scholar] [CrossRef]
- Anonymous. Regulation (EU) No. 1169/2011 of the European parliament and of the council of 25 October 2011. Off. J. Eur. Union 2011, 304, 18–63. [Google Scholar]
- Kanny, G.; De Hauteclocque, C.; Moneret-Vautrin, D.A. Sesame seed and sesame seed oil contain masked allergens of growing importance. Allergy 1996, 51, 952–957. [Google Scholar] [CrossRef] [PubMed]
- Gangur, V.; Kelly, C.; Navuluri, L. Sesame allergy: A growing food allergy of global proportions? Ann. Allergy Asthma Immunol. 2005, 95, 4–11. [Google Scholar] [CrossRef]
- Neering, H.; Vitányi, B.E.; Malten, K.E.; van Ketel, W.G.; van Dijk, E. Allergens in sesame oil contact dermatitis. Acta Derm. Venereol. 1975, 55, 31–34. [Google Scholar]
- Kubo, Y.; Nonaka, S.; Yoshida, H. Contact sensitivity to unsaponifiable substances in sesame oil. Contact Derm. 1986, 15, 215–217. [Google Scholar] [CrossRef]
- Li, L.; Piao, H.; Zheng, M.; Jin, Z.; Zhao, L.; Yan, G. Sesamin attenuates allergic airway inflammation through the suppression of nuclear factor-kappa B activation. Exp. Ther. Med. 2016, 12, 4175–4181. [Google Scholar] [CrossRef] [Green Version]
- Hori, H.; Takayanagi, T.; Kamada, Y.; Shimoyoshi, S.; Ono, Y.; Kitagawa, Y.; Shibata, H.; Nagao, M.; Fujii, W.; Sakakibara, Y. Genotoxicity evaluation of sesamin and episesamin. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 719, 21–28. [Google Scholar] [CrossRef]
- Patisaul, H.B. Endocrine disruption by dietary phyto-oestrogens: Impact on dimorphic sexual systems and behaviours. Proc. Nutr. Soc. 2017, 76, 130–144. [Google Scholar] [CrossRef]
- Christov, R.; Bankova, V.; Tsvetkova, I.; Kujumgiev, A.; Delgado Tejera, A. Antibacterial furofuran lignans from Canary Islands propolis. Fitoterapia 1999, 70, 89–92. [Google Scholar] [CrossRef]
- Rangkaew, N.; Suttisri, R.; Moriyasu, M.; Kawanishi, K. A new acyclic diterpene acid and bioactive compounds from Knema glauca. Arch. Pharm. Res. 2009, 32, 685–692. [Google Scholar] [CrossRef]
- Bussey, R.O.; Sy-Cordero, A.A.; Figueroa, M.; Carter, F.S.; Falkinham, J.O.; Oberlies, N.H.; Cech, N.B. Antimycobacterial furofuran lignans from the roots of Anemopsis californica. Planta Med. 2014, 80, 498–501. [Google Scholar] [CrossRef] [Green Version]
- Hwang, B.; Lee, J.; Liu, Q.H.; Woo, E.R.; Lee, D.G. Antifungal effect of (+)-pinoresinol isolated from Sambucus williamsii. Molecules 2010, 15, 3507–3516. [Google Scholar] [CrossRef]
- Kulik, T.; Buśko, M.; Pszczółkowska, A.; Perkowski, J.; Okorski, A. Plant lignans inhibit growth and trichothecene biosynthesis in Fusarium graminearum. Lett. Appl. Microbiol. 2014, 59, 99–107. [Google Scholar] [CrossRef]
- Wynn, J.P.; Kendrick, A.; Ratledge, C. Sesamol as an inhibitor of growth and lipid metabolism in Mucor circinelloides via its action on malic enzyme. Lipids 1997, 32, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Jacklin, A.; Ratledge, C.; Wynn, J.P. Lipid-to-gibberellin metabolic switching in Fusarium moniliforme via the action of sesamol. Biotechnol. Lett. 2000, 22, 1983–1986. [Google Scholar] [CrossRef]
- Plumridge, A.; Hesse, S.J.A.; Watson, A.J.; Lowe, K.C.; Stratford, M.; Archer, D.B. The weak acid preservative sorbic acid inhibits conidial germination and mycelial growth of Aspergillus niger through intracellular acidification. Appl. Environ. Microbiol. 2004, 70, 3506–3511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schlösser, I.; Prange, A. Antifungal activity of selected natural preservatives against the foodborne molds Penicillium verrucosum and Aspergillus westerdijkiae. FEMS Microbiol. Lett. 2018, 365, fny125. [Google Scholar] [CrossRef]
- Razzaghi-Abyaneh, M.; Rai, M. (Eds.) Antifungal Metabolites from Plants; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–469. [Google Scholar]
- Eagleson, C. Oil Synergist for Insecticides. U.S. Patent 2,202,145, 28 May 1940. [Google Scholar]
- Haller, H.L.; Mcgovran, E.R.; Goodhue, L.D.; Sullivan, W.N. The synergistic action of sesamin with pyrethrum insecticides. J. Org. Chem. 1942, 7, 183–185. [Google Scholar] [CrossRef]
- Parkin, E.A.; Green, A.A. Activation of pyrethrins by sesame oil. Nature 1944, 154, 16–17. [Google Scholar] [CrossRef]
- Simanton, W.A. Sesame Extract Synergisized Insecticides. U.S. Patent No. 2,463,324, 1 March 1949. [Google Scholar]
- Beroza, M. Pyrethrum synergists in sesame oil. Sesamolin, a potent synergist. J. Am. Oil Chem. Soc. 1954, 31, 302–305. [Google Scholar] [CrossRef]
- Tong, F.; Bloomquist, J.R. Plant essential oils affect the toxicities of carbaryl and permethrin against Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2013, 50, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Cabral, M.M.; Azambuja, P.; Gottlieb, O.R.; Garcia, E.S. Effects of some lignans and neolignans on the development and excretion of Rhodnius prolixus. Fitoterapia 2000, 71, 1–9. [Google Scholar] [CrossRef]
- Garcia, E.S.; Azambuja, P. Lignoids in insects: Chemical probes for the study of ecdysis, excretion and Trypanosoma cruzi-triatomine interactions. Toxicon 2004, 44, 431–440. [Google Scholar] [CrossRef]
- Bowers, W.S. Juvenile hormone: Activity of natural and synthetic synergists. Science 1968, 161, 895–897. [Google Scholar] [CrossRef]
- Nawrot, J.; Harmatha, J. Phytochemical feeding deterrents for stored product insect pests. Phytochem. Rev. 2012, 11, 543–566. [Google Scholar] [CrossRef]
- Srivastava, S.; Gupta, M.M.; Prajapati, V.; Tripathi, A.K.; Kumar, S. Sesamin a potent antifeedant principle from Piper mullesua. Phytother. Res. 2001, 15, 70–72. [Google Scholar] [CrossRef]
- Kamikado, T.; Chang, C.F.; Murakoshi, S.; Sakurai, A.; Tamura, S. Isolation and structure elucidation of growth inhibitors on silkworm larvae from Magnolia kobus DC. Agric. Biol. Chem. 1975, 39, 833–836. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Geng, Z.; Guo, S.; Cao, J.; Zhang, Z.; Pang, X.; Chen, Z.; Du, S.; Deng, Z. Antifeedant Activities of Lignans from Stem Bark of Zanthoxylum armatum DC. against Tribolium castaneum. Molecules 2018, 23, 617. [Google Scholar] [CrossRef] [Green Version]
- Kunugi, A.; Yun, S.M.; Kobayashi, A. Growth Inhibitor of insect larvae from Paulownia tomentosa. Planta Med. 2006, 72, 293. [Google Scholar] [CrossRef]
- Li, Y.; Wei, J.; Fang, J.; Lv, W.; Ji, Y.; Aioub, A.A.A.; Zhang, J.; Hu, Z. Insecticidal activity of four lignans Isolated from Phryma leptostachya. Molecules 2019, 24, 1976. [Google Scholar] [CrossRef] [Green Version]
- Cloyd, R.A.; Galle, C.L.; Keith, S.R.; Kalscheur, N.A.; Kemp, K.E. Effect of commercially available plant-derived essential oil products on arthropod pests. J. Econ. Entomol. 2009, 102, 1567–1579. [Google Scholar] [CrossRef]
- Friend, J. Phenolic substances and plant disease. In Biochemistry of Plant Phenolics; Swain, T., Harborne, J.B., Van Sumere, C.F., Eds.; Plenum Press: London, UK, 1979; pp. 557–588. [Google Scholar]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A.; Imperato, F. Role of phenolics in the resistance mechanism of plants against fungal pathogens and insects. In Phytochemintry: Advances in Research; Research Signpost: Trivandrum, India, 2006; pp. 23–67. [Google Scholar]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal. Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dowd, C.; Wilson, I.W.; McFadden, H. Gene expression profile changes in cotton root and hypocotyl tissues in response to infection with Fusarium oxysporum f. sp. vasinfectum. Mol. Plant Microbe Interact. 2004, 17, 654–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, R.N.; Laurentin, H.; Splivallo, R.; Karlovsky, P. Antifungal properties of extracts of sesame (Sesamum indicum). Int. J. Agric. Biol. 2015, 17, 575–581. [Google Scholar] [CrossRef]
- Preisner, M.; Kulma, A.; Zebrowski, J.; Dymińska, L.; Hanuza, J.; Arendt, M.; Starzycki, M.; Szopa, J. Manipulating cinnamyl alcohol dehydrogenase (CAD) expression in flax affects fibre composition and properties. BMC Plant Biol. 2014, 14, 50. [Google Scholar] [CrossRef] [Green Version]
- Danielsen, L.; Lohaus, G.; Sirrenberg, A.; Karlovsky, P.; Bastien, C.; Pilate, G.; Polle, A. Ectomycorrhizal colonization and diversity in relation to tree biomass and nutrition in a plantation of transgenic poplars with modified lignin biosynthesis. PLoS ONE 2013, 8, e59207. [Google Scholar] [CrossRef]
- Bagniewska-Zadworna, A.; Barakat, A.; Łakomy, P.; Smoliński, D.J.; Zadworny, M. Lignin and lignans in plant defence: Insight from expression profiling of cinnamyl alcohol dehydrogenase genes during development and following fungal infection in Populus. Plant Sci. 2014, 229, 111–121. [Google Scholar] [CrossRef]
- Hano, C.; Addi, M.; Bensaddek, L.; Crônier, D.; Baltora-Rosset, S.; Doussot, J.; Maury, S.; Mesnard, F.; Chabbert, B.; Hawkins, S.; et al. Differential accumulation of monolignol-derived compounds in elicited flax (Linum usitatissimum) cell suspension cultures. Planta 2006, 223, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Harmatha, J.; Dinan, L. Biological activities of lignans and stilbenoids associated with plant-insect chemical interactions. Phytochem. Rev. 2003, 2, 321–330. [Google Scholar] [CrossRef]
- Victor, R.S.; Crisótomo, F.R.; Bueno, F.C.; Pagnocca, F.C.; Fernandes, J.B.; Correa, G.A.; Bueno, O.C.; Hebling, M.J.A.; Bacci, J.M.; Vieira, P.C.; et al. Toxicity of synthetic piperonyl compounds to leaf-cutting ants and their symbiotic fungus. Pest Manag. Sci. 2001, 57, 603–608. [Google Scholar] [CrossRef]
- Schroeder, F.C.; del Campo, M.L.; Grant, J.B.; Weibel, D.B.; Smedley, S.R.; Bolton, K.L.; Meinwald, J.; Eisner, T. Pinoresinol: A lignol of plant origin serving for defense in a caterpillar. Proc. Natl. Acad. Sci. USA 2006, 103, 15497–15501. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Li, Q.; Tan, H.; Chen, J.; Xiao, Y.; Ma, R.; Gao, S.; Zerbe, P.; Chen, W.; Zhang, L. Gene-to-metabolite network for biosynthesis of lignans in MeJA-elicited Isatis indigotica hairy root cultures. Front. Plant Sci. 2015, 6, 952. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wu, D.; Wang, Y.; Xie, D. Jasmonate action in plant defense against insects. J. Exp. Bot. 2019, 70, 3391–3400. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Vinas, M.; Alsarrag, A.; Su, L.; Pfohl, K.; Rohlfs, M.; Schäfer, W.; Chen, W.; Karlovsky, P. Bis-naphthopyrone pigments protect filamentous ascomycetes from a wide range of predators. Nat. Commun. 2019, 10, 3579. [Google Scholar] [CrossRef] [Green Version]
- Kakani, V.G.; Reddy, K.R.; Zhao, D.; Sailaja, K. Field crop responses to ultraviolet-B radiation: A review. Agric. For. Meteorol. 2003, 120, 191–218. [Google Scholar] [CrossRef]
- Sytar, O.; Zivcak, M.; Neugart, S.; Toutounchi, P.M.; Brestic, M. Precultivation of young seedlings under different color shades modifies the accumulation of phenolic compounds in Cichorium leaves in later growth phases. Environ. Exp. Bot. 2019, 165, 30–38. [Google Scholar] [CrossRef]
- Yamauchi, S.; Ichikawa, H.; Nishiwaki, H.; Shuto, Y. Evaluation of plant growth regulatory activity of furofuran lignan bearing a 7,9′:7′,9-diepoxy structure using optically pure (+)- and (-)-enantiomers. J. Agric. Food Chem. 2015, 63, 5224–5228. [Google Scholar] [CrossRef] [PubMed]
- Ammar, G.A.; Tryono, R.; Döll, K.; Karlovsky, P.; Deising, H.B.; Wirsel, S.G.R. Identification of ABC transporter genes of Fusarium graminearum with roles in azole tolerance and/or virulence. PLoS ONE 2013, 8, e79042. [Google Scholar] [CrossRef] [Green Version]
- Moeller, L.; Wang, K. Engineering with precision: Tools for the new generation of transgenic crops. Bioscience 2008, 58, 391–401. [Google Scholar] [CrossRef] [Green Version]
- Pickens, L.B.; Tang, Y.; Chooi, Y.H. Metabolic engineering for the production of natural products. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 211–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, Y.; Hirose, S.; Taniguchi, Y.; Moriya, Y.; Yamada, T. Targeted enzyme gene re-positioning: A computational approach for discovering alternative bacterial enzymes for the synthesis of plant-specific secondary metabolites. Metab. Eng. 2019, 9, e00102. [Google Scholar] [CrossRef]
- Xie, Q.; Liu, Z.; Meir, S.; Rogachev, I.; Aharoni, A.; Klee, H.J. Altered metabolite accumulation in tomato fruits by coexpressing a feedback-insensitive AroG and the PhODO1 MYB-type transcription factor. Plant Biotechnol. J. 2016, 14, 2300–2309. [Google Scholar] [CrossRef] [Green Version]
- Renouard, S.; Tribalat, M.A.; Lamblin, F.; Mongelard, G.; Fliniaux, O.; Corbin, C. RNAi-mediated pinoresinol lariciresinol reductase gene silencing in flax (Linum usitatissimum) seed coat: Consequences on lignans and neolignans accumulation. J. Plant Physiol. 2014, 171, 1372–1377. [Google Scholar] [CrossRef]
- Wink, M. Biochemistry, physiology and ecological functions of secondary metabolites. In Biochemistry of Plant Secondary Metabolism, 2nd ed.; Wink, M., Ed.; Wiley-Blackwell Publishing: New York, NY, USA, 2010; pp. 1–17. [Google Scholar]
- Li, D.; Dossa, K.; Zhang, Y.; Wei, X.; Wang, L.; Zhang, Y.; Liu, A.; Zhou, R.; Zhang, X. GWAS uncovers differential genetic bases for drought and salt tolerances in sesame at the germination stage. Genes 2018, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, Y.; Li, D.; Dossa, K.; Wang, M.L.; Zhou, R.; Yu, J.; Zhang, X. Gene expression profiles that shape high and low oil content sesames. BMC Genet. 2019, 20, 45. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, D.; Zhou, R.; Wang, X.; Dossa, K.; Wang, L.; Zhang, Y.; Yu, J.; Gong, H.; Zhang, X.; et al. Transcriptome and metabolome analyses of two contrasting sesame genotypes reveal the crucial biological pathways involved in rapid adaptive response to salt stress. BMC Plant Biol. 2019, 19, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.F.; et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [Green Version]
- Ono, E.; Tanaka, Y. Lignan Glycosidase and Utilization of the Same. U.S. Patent No. 7,935,802, 3 May 2011. [Google Scholar]
- Satake, H.; Ono, E.; Murata, J. Recent advances in the metabolic engineering of lignan biosynthesis pathways for the production of transgenic plant-based foods and supplements. J. Agric. Food Chem. 2013, 61, 11721–11729. [Google Scholar] [CrossRef]
- Satake, H.; Koyama, T.; Bahabadi, S.E.; Matsumoto, E.; Ono, E.; Murata, J. Essences in metabolic engineering of lignan biosynthesis. Metabolites 2015, 5, 270–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayella, A.K.; Trick, H.N.; Wang, W. Enhancing lignan biosynthesis by over-expressing pinoresinol lariciresinol reductase in transgenic wheat. Mol. Nutr. Food. Res. 2007, 51, 1518–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnsson, P.; Kamal-Eldin, A.; Lundgren, L.N.; Aman, P. HPLC method for analysis of secoisolariciresinol diglucoside in flaxseeds. J. Agric. Food Chem. 2000, 48, 5216–5219. [Google Scholar] [CrossRef] [PubMed]
- Popova, I.; Hall, C.; Kubatova, A. Determination of lignans in flaxseed using liquid chromatography with time-of-flight mass spectrometry. J. Chromatogr. A 2009, 1216, 217–229. [Google Scholar] [CrossRef]
- Watson, J.M.; Fusaro, A.F.; Wang, M.B.; Waterhouse, P. RNA silencing platforms in plants. FEBS Lett. 2005, 579, 5982–5987. [Google Scholar] [CrossRef]
- Kim, H.J.; Ono, E.; Morimoto, K.; Yamagaki, T.; Okazawa, A.; Kobayashi, A.; Satake, H. Metabolic engineering of lignan biosynthesis in Forsythia cell culture. Plant Cell Physiol. 2009, 50, 2200–2209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Osbourn, A.; Ma, P. MYB transcription factors as regulators of phenylpropanoid metabolism in plants. Mol. Plant 2015, 8, 689–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behr, M.; Sergeant, K.; Leclercq, C.C.; Planchon, S.; Guignard, C.; Lenouvel, A.; Renaut, J.; Hausman, J.F.; Lutts, S.; Guerriero, G. Insights into the molecular regulation of monolignol-derived product biosynthesis in the growing hemp hypocotyl. BMC Plant Biol. 2018, 18, 1. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.; Xiao, Y.; Lv, Z.; Tan, H.; Chen, R.; Li, Q.; Chen, J.; Wang, Y.; Yin, J.; Zhang, L. APp2/ERF transcription factor, Ii049, positively regulates lignan biosynthesis in Isatis indigotica through activating salicylic acid signaling and lignan/lignin pathway genes. Front. Plant Sci. 2017, 8, 1361. [Google Scholar] [CrossRef] [Green Version]
- Tamagnone, L.; Merida, A.; Parr, A.; Mackay, S.; Culianez-Macia, F.A.; Roberts, K.; Martin, C. The AmMYB308 and AmMYB330 transcription factors from Antirrhinum regulate phenylpropanoid and lignin biosynthesis in transgenic tobacco. Plant Cell 1998, 10, 135–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taskin, K.M.; Ercan, A.G.; Turgut, K. Agrobacterium tumefaciens-mediated transformation of sesame (Sesamum indicum L.). Turk. J. Bot. 1999, 23, 291–296. [Google Scholar]
- Chowdhury, S.; Basu, A.; Kundu, S. A new high-frequency Agrobacterium-mediated transformation technique for Sesamum indicum L. using de-embryonated cotyledon as explant. Protoplasma 2014, 251, 1175–1190. [Google Scholar] [CrossRef]
- Al-Shafeay, A.F.; Ibrahim, A.S.; Nesiem, M.R.; Tawfik, M.S. Establishment of regeneration and transformation system in Egyptian sesame (Sesamum indicum L.) cv Sohag 1. GM Crop. 2011, 2, 182–192. [Google Scholar] [CrossRef]
- Yadav, M.; Chaudhary, D.; Sainger, M.; Jaiwal, P.K. Agrobacterium tumefaciens-mediated genetic transformation of sesame (Sesamum indicum L.). Plant Cell Tiss. Organ Cult. 2010, 103, 377–386. [Google Scholar] [CrossRef]
- Bhattacharyya, J.; Chakraborty, A.; Mitra, J.; Chakraborty, S.; Pradhan, S.; Manna, A.; Sikdar, N.; Sen, S.K. Genetic transformation of cultivated sesame (Sesamum indicum L. cv Rama) through particle bombardment using 5-day-old apical, meristematic tissues of germinating seedlings. Plant Cell Tiss. Organ Cult. 2015, 123, 455–466. [Google Scholar] [CrossRef]
- Ono, E.; Satake, H.; Kim, H.-J. Glucosyltransferase Specific to Position-4 of Furofuran-Type Lignan, and Polynucleotide Encoding Same. EPO Patent Application No. 2 434 011, 28 March 2012. [Google Scholar]
- Alfermann, A.W.; Petersen, M.; Fuss, E. Production of natural products by plant cell biotechnology: Results, problems and perspectives. In Plant Tissue Culture, 100 Years since Gottlieb Haberlandt; Laimer, M., Rücker, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 153–166. [Google Scholar]
- Renouard, S.; Corbin, C.; Drouet, S.; Medvedec, B.; Doussot, J.; Colas, C.; Maunit, B.; Bhambra, A.S.; Gontier, E.; Jullian, N.; et al. Investigation of Linum flavum (L.) hairy root cultures for the production of anticancer aryltetralin lignans. Int. J. Mol. Sci. 2018, 19, 990. [Google Scholar] [CrossRef] [Green Version]
- Karuppusamy, S.A. Review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J. Med. Plants Res. 2009, 3, 1222–1239. [Google Scholar]
- Morimoto, K.; Ono, E.; Kim, H.J.; Okazawa, A.; Kobayashi, A.; Satake, H. The construction of transgenic Forsythia plants: Comparative study of three Forsythia species. Plant Biotechnol. 2011, 28, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, K.; Satake, H. Seasonal alteration in amounts of lignans and their glucosides and gene expression of the relevant biosynthetic enzymes in the Forsythia suspense leaf. Biol. Pharm. Bull. 2013, 36, 1519–1523. [Google Scholar] [CrossRef] [Green Version]
- Guillon, S.; Trémouillaux-Guiller, J.; Pati, P.K.; Rideau, M.; Gantet, P. Hairy root research: Recent scenario and exciting prospects. Curr. Opin. Plant Biol. 2006, 9, 341–346. [Google Scholar] [CrossRef]
- Oostdam, A.; Mol, J.M.; van der Plas, H.W. Establishment of hairy root cultures of Linum flavum producing the lignan 5-methoxypodophyllotoxin. Plant Cell Rep. 1993, 12, 474–477. [Google Scholar] [CrossRef]
- Fuss, E. Lignans in plant cell and organ culture: An overview. Phytochem. Rev. 2003, 2, 307–320. [Google Scholar] [CrossRef]
- Mohagheghzadeh, A.; Schmidt, T.J.; Alfermann, A.W. Arylnaphthalene lignans from in vitro cultures of Linum austriacum. J. Nat. Prod. 2002, 65, 69–71. [Google Scholar] [CrossRef]
- Ream, W. Agrobacterium tumefaciens and A. rhizogenes use different proteins to transport bacterial DNA into the plant cell nucleus. Microb. Biotechnol. 2009, 2, 416–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, R.G.F.; Jacobsen, E.; Witholt, B.; Feenstra, W.J. Efficient transformation of potato (Solanum tuberosum L.) using a binary vector in Agrobacterium rhizogenes. Theor. Appl. Genet. 1989, 78, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Vilaine, F.; Casse-Delbart, F. A new vector derived from Agrobacterium rhizogenes plasmids: A micro-Ri plasmid and its use to construct a mini-Ri plasmid. Gene 1987, 55, 105–114. [Google Scholar] [CrossRef]
- Furumoto, T.; Sato, R. Biosynthesis of anthraquinone derivatives in a Sesamum indicum hairy root culture. Biosci. Biotech. Biochem. 2017, 81, 1868–1873. [Google Scholar] [CrossRef] [Green Version]










| Lignan | MW | Monoisotopic Mass | UV Maxima | Extinction Coefficient [g−1 L] | Content in Seeds [mg/100 g] | Reference |
|---|---|---|---|---|---|---|
| Sesamin | 354.35 | 354.1103 | 287/236 | 23.03/26.01 4 | 77–930 | [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67] |
| Sesamolin | 370.35 | 370.1053 | 288/235 | 21.79/24.85 4 | 61–530 | [53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72] |
| Sesaminol | 370.40 | 370.1053 | 238/295 | 3.99/3.85 5 | 0–1.4 | [73,74] |
| Sesamolinol | 372.40 | 372.1209 | 231/287 | 3.95/3.80 5 | 6–29 | [53,75] |
| Pinoresinol | 358.38 | 358.1416 | 232/280 | - | 29–38 | [52,76,77,78] |
| Sesamol 1 | 138.12 | 138.0316 | 296/233 | 29.74/21.18 4 | 0–30 1 | [62,63,79,80,81] |
| Lariciresinol | 360.40 | 360.1572 | 230/280 | - | 0.8 3 | [77,78,82] |
| Matairesinol | 358.40 | 358.1416 | 230/282 | - | 0.4 3 | [77] |
| Piperitol | 356.40 | 356.1259 | - | - | 1.3 3 | [78] |
| Episesamin 2 | 354.40 | 354.1103 | - | - | 9–270 | [54,73] |
| Samin | 250.25 | 250.0841 | 287/238 | - | 50 2 | [83,84] |
| Source | (+)-Sesamin | (+)-Sesamolin | (+)-Sesan-Golin | (+)-ala-Tumin | (+)-2-epi-Sesalatin | (+)-7′-epi-Sesantalin |
|---|---|---|---|---|---|---|
| S. alatum | [87] 1 | [87] 2 | [88] | [89] | [90] | |
| S. angolense | [66] | [66] | [91] | |||
| S. angustifolium | [66] | [66] | [87] | |||
| S. orientalevar. malabaricum | [66] 3 | [66] 3 | ||||
| S. calycinum | [66] | [66] | ||||
| S. latifolium | [66] | [87] 4 | ||||
| S. radiatum | [66] | [72] 4,5 | [92] | [92] | ||
| S. schinzianum | [72] 6 | [72] 6 | ||||
| S. mulayanum | [93] 7 | [93] 7 | ||||
| S. laciniatum | [93] 8 | [93] 8 | ||||
| S. capense | [66] 9 | |||||
| S. pedalioides | [66] 9 |
| Lignan | MW | Monoisotopic Mass | Content in Seeds [mg/100 g] | Reference |
|---|---|---|---|---|
| Sesaminol monoglucoside | 532.5 | 532.1581 | 5–20 | [53,98,99] |
| Sesaminol diglucoside | 694.6 | 694.2109 | 8–18 | [53,98,99,100] |
| Sesaminol triglucoside | 830.7 | 830.2481 | 14–91 | [53,99,100] |
| Sesamolinol diglucoside | 696.6 | 696.2265 | 5–232 | [100] 1 |
| Pinoresinol diglucoside 2 | 682.7 | 682.2473 | 1.4–2.1 | [76,98] |
| Pinoresinol triglucoside | 844.8 | 844.3001 | 5.22 | [98] |
| Tissue | Lignans (µg/g) | Reference | |
|---|---|---|---|
| Sesamin | Sesamolin | ||
| Callus | 63–460 | 270–950 | [224,225,226] |
| Leaves | 2.6 | - | [232] |
| Leaves, stem, roots, and flower | detected | detected | [232] * |
| Roots | 0–220 | not detected | [231] |
| Hairy roots | 0–75 | not detected | [231] |
| Protein Name/Accession | Type of Enzyme | Function in the Pathway | EC Number |
|---|---|---|---|
| XP_011080883 * | Dirigent protein | Impart stereoselectivity on the phenoxy radical-coupling reaction | - |
| CYP81Q1 | Cytochrome P450 | Piperitol/sesamin synthase. Formation of dual methylenedioxy bridge | 1.14.19.74 |
| CYP92B14 | Cytochrome P450 | Sesamolin/sesaminol synthase.Converts sesamin to sesamolin or sesaminol | 1.14.19.- |
| UGT71A9 | Glycosyltransferase | Catalyzes the glucosylation at the 2-hydroxyl group of sesaminol | 2.4.1.- |
| UGT94D1/UGT94AA2 | Glycosyltransferase | Catalyzes the β1→6 glucosylation toward the sugar moiety of sesaminol 2-O-glucoside | 2.4.1.- |
| UGT94AG1 | Glycosyltransferase | Catalyzes the β1→2-O-glucosylation of sesaminol mono glucoside and sesaminol 2-O-β-d-glucosyl-(β1→6)-O-β-d-glucoside | 2.4.1.- |
| XP_011092597 (SinPLR2) * | Bifunctional pinoresinol-lariciresinol reductase | Conversion of pinoresinol into lariciresinol and lariciresinol into secoisolariciresinol | 1.23.1.1 |
| XP_011094269 * | Secoisolariciresinol dehydrogenase-like | Conversion of secoisolariciresinol into matairesinol | 1.1.1.331 |
| NCBI Accession Number or Gene ID | Gene Name | GC Content in Exons (%) | Nc Value * | Number of Introns | Linkage Group ** |
|---|---|---|---|---|---|
| LOC105164033 | ---- | 52.0 | 56.06 | 0 | LG6 |
| AB194714 | CYP81Q1 | 51.2 | 57.39 | 1 | LG15 |
| LC199944 | CYP92B14 | 43.1 | 52.34 | 1 | LG2 |
| AB194716 | CYP81Q3 | 51.3 | 57.02 | 0 | (S. alatum) |
| AB293960 | UGT71A9 | 47.6 | 59.01 | 0 | LG16 |
| AB333799 | UGT94D1 | 49.8 | 53.13 | 0 | LG4 |
| LC484014 | UGT94AA2 | 53.6 | 53.44 | 0 | LG7 |
| LC484013 | UGT94AG1 | 40.6 | 50.50 | 0 | LG10 |
| LOC105172736 | SinPLR2 | 45.2 | 57.93 | 3 | LG10 |
| LOC105174016 | ---- | 46.6 | 56.82 | 1 | LG11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andargie, M.; Vinas, M.; Rathgeb, A.; Möller, E.; Karlovsky, P. Lignans of Sesame (Sesamum indicum L.): A Comprehensive Review. Molecules 2021, 26, 883. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26040883
Andargie M, Vinas M, Rathgeb A, Möller E, Karlovsky P. Lignans of Sesame (Sesamum indicum L.): A Comprehensive Review. Molecules. 2021; 26(4):883. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26040883
Chicago/Turabian StyleAndargie, Mebeaselassie, Maria Vinas, Anna Rathgeb, Evelyn Möller, and Petr Karlovsky. 2021. "Lignans of Sesame (Sesamum indicum L.): A Comprehensive Review" Molecules 26, no. 4: 883. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26040883