Insecticidal Activity and Free Radical Scavenging Properties of Isolated Phytoconstituents from the Saudi Plant Nuxia oppositifolia (Hochst.)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Isolated Compounds
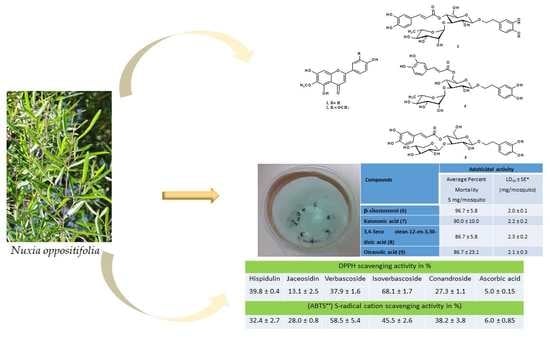
2.2. Adulticidal Activity
2.3. Free Radical Scavenging Activity
3. Materials and Methods
3.1. Apparatus and Chemicals
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Adulticidal Activity
3.5. Radical Scavenging Activity
3.5.1. DPPH (2, 2-diphenyl-1-picrylhydrazyl) Scavenging Activity
3.5.2. ABTS•+ Radical Cation Scavenging Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zanotto, P.M.A.; Leite, L.C.C. The challenges imposed by Dengue, Zika, and Chikungunya to Brazil. Front Immunol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- War, A.R.; Paulraj, M.G.; Ahmad, T.; Buhroo, A.; Hussain, B.; Ignacimuthu, S.; Sharma, H.C. Mechanisms of plant defense against insect herbivores. Plant Signal Behav. 2012, 7, 1306–1320. [Google Scholar] [CrossRef] [Green Version]
- Jensen, S.R.; Ravnkilde, L.; Schripsema, J. Unedoside derivatives in Nuxia and their biosynthesis. Phytochemistry 1998, 47, 1007–1011. [Google Scholar] [CrossRef]
- Jensen, S.R. Chemotaxonomy of the genus Nuxia (Buddlejaceae). Stud. Plant Sci. 1999, 6, 379–382. [Google Scholar]
- Al-Massarani, S.M.; El-Gamal, A.A.; Parvez, M.K.; Al-Dosari, M.S.; Al-Said, M.S.; Abdel-Kader, M.S.; Basudan, O.A. New cytotoxic seco-type triterpene and labdane-type diterpenes from Nuxia oppositifolia. Molecules 2017, 22, 389. [Google Scholar] [CrossRef] [Green Version]
- Mambu, L.; Grellier, P.; Florent, L.; Joyeau, R.; Ramanitrahasimbola, D.; Rasoanaivo, P.; Frappier, F. Clerodane and labdane diterpenoids from Nuxia sphaerocephala. Phytochemistry 2006, 67, 444–451. [Google Scholar] [CrossRef]
- Boaduo, N.K.K.; Katerere, D.; Eloff, J.N.; Naidoo, V. Evaluation of six plant species used traditionally in the treatment and control of diabetes mellitus in South Africa using in vitro methods. Pharm. Biol. 2014, 52, 756–761. [Google Scholar] [CrossRef] [Green Version]
- Jonville, M.C.; Kodja, H.; Strasberg, D.; Pichette, A.; Ollivier, E.; Frédérich, M.; Angenot, L.; Legault, J. Antiplasmodial, anti- inflammatory and cytotoxic activities of various plant extracts from the Mascarene Archipelago. J. Ethnopharmacol. 2011, 136, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Jonville, M.C.; Kodja, H.; Humeau, L.; Fournel, J.; De Mol, P.; Cao, M.; Angenot, L.; Frédérich, M. Screening of medicinal plants from Reunion Island for antimalarial and cytotoxic activity. J. Ethnopharmacol. 2008, 120, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, A.S.; Hidayathulla, S.; Rehman, M.T.; ElGamal, A.A.; Al-Massarani, S.M.; Razmovski-Naumovski, V.; Alqahtani, M.S.; El Dib, R.A.; AlAjmi, M.F. Alpha-amylase and alpha-glucosidase enzyme inhibition and antioxidant potential of 3-oxolupenal and katononic acid isolated from Nuxia oppositifolia. Biomolecules 2019, 10, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.C.; Hsu, K.C.; Chiou, L.C.; Tseng, H.J.; Huang, W.J. Total synthesis and metabolic stability of hispidulin and its d-labelled derivative. Molecules 2017, 22, 1897. [Google Scholar] [CrossRef] [Green Version]
- Jeong, M.A.; Lee, K.W.; Yoon, D.Y.; Lee, H.J. Jaceosidin, a pharmacologically active flavone derived from Artemisia argyi, inhibits phorbol-ester-induced upregulation of COX-2 and MMP-9 by blocking phosphorylation of ERK-1 and −2 in cultured human mammary epithelial cells. Ann. N. Y. Acad. Sci. 2007, 1095, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, A.; Pati, S.; Minervini, F.; D’Antuono, I.; Linsalata, V.; Lattanziom, V. Verbascoside, isoverbascoside, and their derivatives recovered from olive mill wastewater as possible food antioxidants. J. Agric. Food Chem. 2012, 60, 1822–1829. [Google Scholar] [CrossRef]
- Xie, J.; Tan, F.; Zhu, J.; Yue, C.; Li, Q. Separation, purification and quantification of verbascoside from Penstemon barbatus (Cav.) Roth. Food Chem. 2012, 135, 2536–2541. [Google Scholar] [CrossRef]
- Kawada, T.; Asano, R.; Makino, K.; Sakuno, T. Synthesis of conandroside: A dihydroxyphenylethyl glycoside from Conandron ramaidioides. Eur. J. Org. Chem. 2000, 2000, 2723–2727. [Google Scholar] [CrossRef]
- Abdul Rahuman, A.; Gopalakrishnan, G.; Venkatesan, P.; Geetha, K. Isolation and identification of mosquito larvicidal compound from Abutilon indicum (Linn.) Sweet. Parasitol. Res. 2008, 102, 981–988. [Google Scholar] [CrossRef]
- Tabanca, N.; Ali, Z.; Bernier, U.R.; Epsky, N.D.; Nalbantsoy, A.; Khan, I.A.; Ali, A. Bioassay-guided isolation and identification of Aedes aegypti larvicidal and biting deterrent compounds from Veratrum lobelianum. Open Chem. 2018, 16, 324–332. [Google Scholar] [CrossRef]
- Amin, E.; Radwan, M.M.; El-Hawary, S.S.; Fathy, M.M.; Mohammed, R.; Becnel, J.J.; Khan, I. Potent insecticidal secondary metabolites from the medicinal plant Acanthus montanus. Rec. Nat. Prod. 2012, 6, 301–305. [Google Scholar]
- Senathilake, K.S.; Karunanayake, E.H.; Samarakoon, S.R.; Tennekoon, K.H.; de Silva, E.D.; Adhikari, A. Oleanolic acid from antifilarial triterpene saponins of Dipterocarpus zeylanicus induces oxidative stress and apoptosis in filarial parasite Setaria digitata in vitro. Exp. Parasitol. 2017, 177, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Gutiérrez-Rebolledo, G.A.; Garduño-Siciliano, L.; Chávez-Rueda, A.K.; Siordia-Reyes, A.G.; Zamilpa, A.; Jiménez-Arellanes, M.A. In Vivo anti-arthritic and antioxidant effects from the standardized ethanolic extract of Moussonia deppeana. Rev. Bras. Farmacogn. 2018, 28, 198–206. [Google Scholar] [CrossRef]
- Vertuani, S.; Beghelli, E.; Scalambra, E.; Malisardi, G.; Copetti, S.; Roberto Dal Toso, R.; Baldisserotto, A.; Manfredini, S. Activity and stability studies of verbascoside, a novel antioxidant, in dermo-cosmetic and pharmaceutical topical formulations. Molecules 2011, 16, 7068–7080. [Google Scholar] [CrossRef]
- Zheng, R.L.; Wang, P.F.; Li, J.; Liu, Z.M.; Jia, Z.J. Inhibition of the autoxidation of linoleic acid by phenylpropanoid glycosides from Pedicularis in micelles. Chem. Phys. Lipids 1993, 65, 151–154. [Google Scholar] [CrossRef]
- Koleva, I.I.; van Beek, T.A.; Linssen, J.P.; de Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- Nonaka, G.; Nishioka, I. Bitter phenylpropanoid glycosides from Conandron ramoidioides. Phytochemistry 1977, 16, 1265–1267. [Google Scholar] [CrossRef]
- Pridgeon, J.W.; Meepagala, K.M.; Becnel, J.J.; Clark, G.G.; Pereira, R.M.; Linthicum, K.J. Structure-activity relationships of 33 piperidines as toxicants against female adults of Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2007, 44, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masi, M.; van der Westhuyzen, A.E.; Tabanca, N.; Evidente, M.; Cimmino, A.; Green, I.R.; Bernier, U.R.; Becnel, J.J.; Bloomquist, J.R.; van Otterlo, W.A.L.; et al. Sarniensine, a mesembrine-type alkaloid isolated from Nerine sarniensis, an indigenous South African Amaryllidaceae, with larvicidal and adulticidal activities against Aedes aegypti. Fitoterapia 2017, 116, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Mothana, R.A.; Khaled, J.M.; El-Gamal, A.A.; Noman, O.M.; Kumar, A.; Alajmi, M.F.; Al-Rehaily, A.J.; Al-Said, M.S. Comparative evaluation of cytotoxic, antimicrobial and antioxidant activities of the crude extracts of three Plectranthus species grown in Saudi Arabia. Saudi Pharm. J. 2019, 27, 162–170. [Google Scholar] [CrossRef]
- Alqahtani, A.S.; Herqash, R.N.; Noman, O.M.; Nasr, F.A.; Alyhya, N.; Anazi, S.H.; Farooq, M.; Ullah, R. In Vitro Antioxidant, cytotoxic activities, and phenolic profile of Senecio glaucus from Saudi Arabia. Evid.-Based Complement. Altern. Med. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]


| Compounds | Adulticidal Activity | ||
|---|---|---|---|
| Average Percent Mortality 5 μg/mosquito | LD50 (95% CI) * (μg/mosquito) | R2 | |
| Hispidulin (1) | 70.0 ± 26.5 | - | - |
| Jaceosidin (2) | 63.3 ± 20.8 | - | - |
| β-sitostosterol (6) | 96.7 ± 5.8 | 2.10 (1.90–2.09) | 0.9820 |
| Katononic acid (7) | 90.0 ± 10.0 | 2.22 (1.89–2.53) | 0.9424 |
| 3,4-Seco olean-12-en-3,30-dioic acid (8) | 86.7 ± 5.8 | 2.08 (n.d.*) | 0.7976 |
| Oleanolic acid (9) | 86.7 ± 23.1 | 2.15 (1.64-2.67) | 0.8642 |
| 3-Oxolupenal (10) | 76.7 ± 5.8 | - | - |
| Ifflaionic acid (11) | 73.3 ± 37.9 | - | - |
| Ursolic acid (12) | 73.3 ± 20.8 | - | - |
| 3-Oxolupenol (13) | 70.0 ± 10.0 | - | - |
| Plectranthoic acid (14) | 63.3 ± 5.8 | - | - |
| Maslinic acid (15) | 66.7 ± 15.3 | - | - |
| Asiatic acid (16) | 56.7 ± 15.3 | - | - |
| Untreated | 0 | - | - |
| Solvent control (acetone only) | 0 | - | - |
| 0.15 ng permethrin | 37.0 ± 6.0 | - | - |
| 0.23 ng permethrin | 63.0 ± 5.0 | - | - |
| 2.37 ng permethrin | 100 ± 0 | - | - |
| Sample | DPPH-Radical Scavenging Activity in % | |||||
|---|---|---|---|---|---|---|
| 25 | 50 | 100 | 500 | 1000 | IC50 | |
| (µg/mL) | ||||||
| Hispidulin (1) | 45.7 ± 0.3 | 56.9 ± 0.2 | 67.9 ± 0.1 | 72.5 ± 0.4 | 79.2 ± 0.1 | 34.5 ± 0.8 |
| Jaceosidin (2) | 44.5 ± 0.3 | 53.7 ± 0.1 | 58.4 ± 0.2 | 71.3 ± 0.2 | 79.8 ± 0.1 | 39.8 ± 0.4 |
| Verbascoside (3) | 42.3 ± 2.4 | 57.3 ± 0.7 | 64.7 ± 0.2 | 67.9 ± 0.1 | 79.2 ± 0.7 | 37.9 ± 1.6 |
| Isoverbascoside (4) | 42.3 ± 0.6 | 44.8 ± 1.6 | 59.1 ± 0.5 | 70.5 ± 0.6 | 75.7 ± 0.4 | 68.1 ± 1.7 |
| Conandroside (5) | 49.3 ± 0.2 | 55.8 ± 1.9 | 65.5 ± 0.5 | 71.8 ± 0.2 | 79.8 ± 1.2 | 27.3 ± 1.1 |
| l-Ascorbic acid | 80.7 ± 2.0 | 85.1 ± 1.3 | 85 ± 1.2 | 88.7 ± 2.4 | 90.7 ± 1.4 | 5.0 ± 0.15 |
| (ABTS•+) Radical Cation Scavenging Activity in % | ||||||
| Hispidulin (1) | 47.7 ± 2.7 | 55.3 ± 0.7 | 64.4 ± 0.5 | 69.5 ± 0.7 | 75.7 ± 1.7 | 32.4 ± 2.7 |
| Jaceosidin (2) | 49.2 ± 0.5 | 56.3 ± 1 | 63.1 ± 0.4 | 70.6 ± 0.5 | 77.1 ± 0.1 | 28.0 ± 0.8 |
| Verbascoside (3) | 43.7 ± 1.2 | 49.3 ± 0.5 | 54.6 ± 0.5 | 63.1 ± 0.7 | 76.5 ± 0.4 | 58.5 ± 5.4 |
| Isoverbascoside (4) | 44.2 ± 0.7 | 51.4 ± 0.9 | 51.4 ± 1.9 | 66.9 ± 0.5 | 73.7 ± 0.3 | 45.5 ± 2.6 |
| Conandroside (5) | 47.4 ± 1.5 | 52.3 ± 0.2 | 60.6 ± 0.7 | 65.3 ± 0.9 | 75.8 ± 0.2 | 38.2 ± 3.8 |
| l-Ascorbic acid | 80.7 ± 2.4 | 81.2 ± 2.1 | 84.2 ± 1.9 | 87.2 ± 2.4 | 88.7 ± 2.1 | 6.0 ± 0.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Massarani, S.M.; El-Gamal, A.A.; Al-Rehaily, A.J.; Al-Sheddi, E.S.; Al-Oqail, M.M.; Farshori, N.N.; Estep, A.S.; Tabanca, N.; Becnel, J.J. Insecticidal Activity and Free Radical Scavenging Properties of Isolated Phytoconstituents from the Saudi Plant Nuxia oppositifolia (Hochst.). Molecules 2021, 26, 914. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26040914
Al-Massarani SM, El-Gamal AA, Al-Rehaily AJ, Al-Sheddi ES, Al-Oqail MM, Farshori NN, Estep AS, Tabanca N, Becnel JJ. Insecticidal Activity and Free Radical Scavenging Properties of Isolated Phytoconstituents from the Saudi Plant Nuxia oppositifolia (Hochst.). Molecules. 2021; 26(4):914. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26040914
Chicago/Turabian StyleAl-Massarani, Shaza M., Ali A. El-Gamal, Adnan J. Al-Rehaily, Ebtesam S. Al-Sheddi, Mai M. Al-Oqail, Nida N. Farshori, Alden S. Estep, Nurhayat Tabanca, and James J. Becnel. 2021. "Insecticidal Activity and Free Radical Scavenging Properties of Isolated Phytoconstituents from the Saudi Plant Nuxia oppositifolia (Hochst.)" Molecules 26, no. 4: 914. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26040914