Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy
Abstract
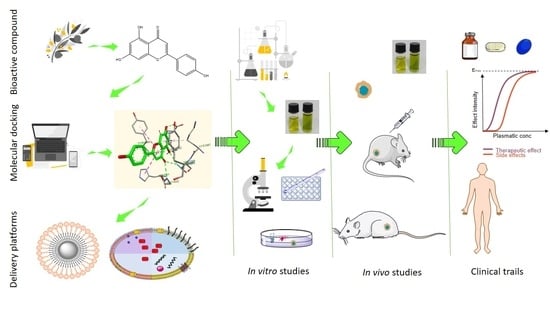
:1. Introduction
2. Natural Compounds Recognized as Chemotherapeutic Agents
2.1. Natural Compounds Approved as Chemotherapeutic Agents
2.2. Natural Compounds with Anticancer Potential
3. Natural Compounds Effective as Chemopreventive Agents
4. Natural Compounds as Sensitizers in Drug Resistance
5. Efficient Platforms for the Incorporation and Delivery of Natural Compounds
6. Conclusions
Funding
Conflicts of Interest
References
- Veeresham, C. Natural products derived from plants as a source of drugs. J. Adv. Pharm. Technol. Res. 2012, 3, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Koparde, A.A.; Doijad, R.C.; Magdum, C.S. Natural Products in Drug Discovery. Available online: https://www.intechopen.com/books/pharmacognosy-medicinal-plants/natural-products-in-drug-discovery (accessed on 16 November 2020).
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cragg, G.M.; Pezzuto, J.M. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med. Princ. Pract. 2016, 25 (Suppl 2), 41–59. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [Green Version]
- de Melo, F.H.M.; Oliveira, J.S.; Sartorelli, V.O.B.; Montor, W.R. Cancer chemoprevention: Classic and epigenetic mechanisms inhibiting tumorigenesis. What have we learned so far? Front Oncol. 2018, 8, 644. [Google Scholar] [CrossRef] [Green Version]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, V.C.; Dellaire, G.; Rupasinghe, H.P.V. Plant flavonoids in cancer chemoprevention: Role in genome stability. J. Nutr. Biochem. 2017, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gullett, N.P.; Ruhul Amin, A.R.; Bayraktar, S.; Pezzuto, J.M.; Shin, D.M.; Khuri, F.R.; Aggarwal, B.B.; Surh, Y.J.; Kucuk, O. Cancer prevention with natural compounds. Semin. Oncol. 2010, 37, 258–281. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [Green Version]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Meier, B.P.; Lappas, C.M. The Influence of safety, efficacy, and medical condition severity on natural versus synthetic drug preference. Med. Deci. Making 2016, 36, 1011–1019. [Google Scholar] [CrossRef]
- Aung, T.N.; Qu, Z.; Kortschak, R.D.; Adelson, D.L. Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int. J. Mol. Sci. 2017, 18, 656. [Google Scholar] [CrossRef]
- Lin, S.R.; Chang, C.H.; Hsu, C.F.; Tsai, M.J.; Cheng, H.; Leong, M.K.; Sung, P.J.; Chen, J.C.; Weng, C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef] [Green Version]
- Calixto, J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Cienc. 2019, 91. [Google Scholar] [CrossRef]
- Mohr, K.I. History of antibiotics research. Curr. Top Microbiol. Immunol. 2016, 398, 237–272. [Google Scholar] [CrossRef]
- McGowan, J.V.; Chung, R.; Maulik, A.; Piotrowska, I.; Walker, J.M.; Yellon, D.M. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc. Drugs Ther. 2017, 31, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perveen, F.; Arshad, N.; Qureshi, R.; Nowsherwan, J.; Sultan, A.; Nosheen, B.; Rafique, H. Electrochemical, spectroscopic and theoretical monitoring of anthracyclines’ interactions with DNA and ascorbic acid by adopting two routes: Cancer cell line studies. PLoS One 2018, 13, e0205764. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Rawat, P.; Singh, P.K. Adverse drug reactions of anticancer drugs derived from natural sources. Food Chem. Toxicol. 2019, 123, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.P.; Gerriets, V. Bleomycin. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK555895/ (accessed on 2 December 2020).
- Kellogg, E.H.; Hejab, N.M.A.; Howes, S.; Northcote, P.; Miller, J.H.; Díaz, J.F.; Downing, K.H.; Nogales, E. Insights into the distinct mechanisms of action of Taxane and non-Taxane microtubule stabilizers from Cryo-EM structures. J. Mol. Biol. 2017, 429, 633–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moudi, M.; Go, R.; Yien, C.Y.; Nazre, M. Vinca alkaloids. In.t J Prev. Med. 2013, 4, 1231–1235. [Google Scholar]
- Wang, Y.R.; Chen, S.F.; Wu, C.C.; Liao, Y.W.; Lin, T.S.; Liu, K.T.; Chen, Y.S.; Li, T.K.; Chien, T.C.; Chan, N.L. Producing irreversible topoisomerase II-mediated DNA breaks by site-specific Pt(II)-methionine coordination chemistry. Nucleic Acids Res. 2017, 45, 10861–10871. [Google Scholar] [CrossRef] [Green Version]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-inflammatory action and mechanisms of resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the car-diovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [Green Version]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.J.; Prickril, B.; Rasooly, A. Mechanisms of phytonutrient modulation of cyclooxygenase-2 (COX-2) and inflammation related to cancer. Nutr. Cancer. 2018, 70, 350–375. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Ji, C.; Mayfield, J.E.; Goel, A.; Xiao, J.; Dixon, J.E.; Guo, X. Ancient drug curcumin impedes 26S proteasome activity by direct inhibition of dual-specificity tyrosine-regulated kinase 2. Proc. Natl. Acad. Sci. USA 2018, 115, 8155–8160. [Google Scholar] [CrossRef] [Green Version]
- Kunnumakkara, A.B.; Harsha, C.; Banik, K.; Vikkurthi, R.; Sailo, B.L.; Bordoloi, D.; Gupta, S.C.; Aggarwal, B.B. Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert Opin. Drug Metab. Toxicol. 2019, 15, 705–733. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Y.F. Natural compounds as anticancer agents: Experimental evidence. World J. Exp. Med. 2012, 2, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Lazzeroni, M.; Guerrieri-Gonzaga, A.; Gandini, S.; Johansson, H.; Serrano, D.; Cazzaniga, M.; Aristarco, V.; Macis, D.; Mora, S.; Caldarella, P.; et al. A presurgical study of lecithin formulation of green tea extract in women with early breast cancer. Cancer Prev. Res. 2017, 10, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Musial, C.; Kuban-Jankowska, A.; Gorska-Ponikowska, M. Beneficial properties of green tea catechins. Int. J. Mol. Sci. 2020, 21, 1744. [Google Scholar] [CrossRef] [Green Version]
- Chow, H.H.; Hakim, I.A.; Vining, D.R.; Crowell, J.A.; Ranger-Moore, J.; Chew, W.M.; Celaya, C.A.; Rodney, S.R.; Hara, Y.; Alberts, D.S. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin. Cancer Res. 2005, 1112, 4627–4633. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Farias, M.; Carrasco-Pozo, C. The anti-cancer effect of quercetin: Molecular implications in cancer metabolism. Int. J. Mol. Sci. 2019, 20, 3177. [Google Scholar] [CrossRef] [Green Version]
- Kedhari Sundaram, M.; Raina, R.; Afroze, N.; Bajbouj, K.; Hamad, M.; Haque, S.; Hussain, A. Quercetin modulates signaling pathways and induces apoptosis in cervical cancer cells. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, A.B.; Mir, H.; Kapur, N.; Gales, D.N.; Carriere, P.P.; Singh, S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 2018, 16, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.M.; Deng, X.T.; Zhou, J.; Li, Q.P.; Ge, X.X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef]
- Sturza, A.; Pavel, I.; Ancușa, S.; Danciu, C.; Dehelean, C.; Duicu, O.; Muntean, D. Quer-cetin exerts an inhibitory effect on cellular bioenergetics of the B164A5 murine mela-noma cell line. Mol. Cell Biochem. 2018, 447, 103–109. [Google Scholar] [CrossRef]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting multiple signaling pathways in cancer: The rutin therapeutic approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef] [PubMed]
- Ben Sghaier, M.; Pagano, A.; Mousslim, M.; Ammari, Y.; Kovacic, H.; Luis, J. Rutin inhibits proliferation, attenuates superoxide production and decreases adhesion and migration of human cancerous cells. Biomed. Pharmacother. 2016, 84, 1972–1978. [Google Scholar] [CrossRef]
- Guo, Y.; Zhu, H.; Weng, M.; Wang, C.; Sun, L. Chemopreventive effect of Betulinic acid via mTOR-Caspases/Bcl2/Bax apoptotic signaling in pancreatic cancer. BMC Complement Med. Ther. 2020, 20, 178. [Google Scholar] [CrossRef]
- Kumar, P.; Bhadauria, A.S.; Singh, A.K.; Saha, S. Betulinic acid as apoptosis activator: Molecular mechanisms, mathematical modeling and chemical modifications. Life Sci. 2018, 209, 24–33. [Google Scholar] [CrossRef]
- Gheorgheosu, D.; Duicu, O.; Dehelean, C.; Soica, C.; Muntean, D. Betulinic acid as a potent and complex antitumor phytochemical: A minireview. Anticancer Agents Med. Chem. 2014, 14, 936–945. [Google Scholar] [CrossRef]
- Soica, C.; Danciu, C.; Savoiu-Balint, G.; Borcan, F.; Ambrus, R.; Zupko, I.; Bojin, F.; Coricovac, D.; Ciurlea, S.; Avram, S.; et al. Betulinic acid in complex with a gamma-cyclodextrin derivative decreases proliferation and in vivo tumor development of non-metastatic and metastatic B164A5 cells. Int. J. Mol. Sci. 2014, 15, 8235–8255. [Google Scholar] [CrossRef] [Green Version]
- Butler, M.S.; Robertson, A.A.; Cooper, M.A. Natural product and natural product derived drugs in clinical trials. Nat. Prod. Rep. 2014, 31, 1612–1661. [Google Scholar] [CrossRef]
- Circioban, D.; Ledeti, A.; Vlase, G.; Moaca, A.; Ledeti, I.; Farcas, C.; Vlase, T.; Dehelean, C. Thermal degradation, kinetic analysis and evaluation of biological activity on hu-man melanoma for artemisinin. J. Therm. Anal. Calorim. 2018, 134, 741–748. [Google Scholar] [CrossRef]
- Wong, Y.K.; Xu, C.; Kalesh, K.A.; He, Y.; Lin, Q.; Wong, W.S.F.; Shen, H.M.; Wang, J. Artemisinin as an anticancer drug: Recent advances in target profiling and mechanisms of action. Med. Res. Rev. 2017, 37, 1492–1517. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Guan, Y. Artemisinin induces selective and potent anticancer effects in drug resistant breast cancer cells by inducing cellular apoptosis and autophagy and G2/M cell cycle arrest. J. BUON. 2020, 25, 1330–1336. [Google Scholar]
- Jiang, F.; Zhou, J.Y.; Zhang, D.; Liu, M.H.; Chen, Y.G. Artesunate induces apoptosis and autophagy in HCT116 colon cancer cells, and autophagy inhibition enhances the artesunate-induced apoptosis. Int. J. Mol. Med. 2018, 42, 1295–1304. [Google Scholar] [CrossRef] [Green Version]
- Circioban, D.; Ledeti, I.; Suta, L.M.; Vlase, G.; Ledeti, A.; Vlase, T.; Varut, R.; Sbarcea, L.; Trandafirescu, C.; Dehelean, C. Instrumental analysis and molecular modelling of in-clusion complexes containing artesunate. J. Therm. Anal. Calorim. 2020, 142, 1951–1961. [Google Scholar] [CrossRef]
- Dai, D.; Zhang, C.F.; Williams, S.; Yuan, C.S.; Wang, C.Z. Ginseng on cancer: Potential role in modulating inflammation-mediated angiogenesis. Am. J. Chin. Med. 2017, 45, 13–22. [Google Scholar] [CrossRef]
- Unlu, A.; Nayir, E.; Kirca, O.; Ay, H.; Ozdogan, M. Ginseng and cancer. J. BUON. 2016, 21, 1383–1387. [Google Scholar]
- Kim, J.; Yoo, J.M.; Kim, J.S.; Kim, S.G.; Park, J.E.; Seok, Y.M.; Son, J.H.; Kim, H.J. Anticancer effect of mountain ginseng. Evid. Based Complement Alternat. Med. 2020. [CrossRef]
- Yoo, H.; Kim, J.M.; Jo, E.; Cho, C.; Lee, S.; Kang, H.S.; Lee, M.; Yang, P.; Jang, I. Modified Panax ginseng extract regulates autophagy by AMPK signaling in A549 human lung cancer cells. Oncol. Rep. 2017, 37, 3287–3296. [Google Scholar] [CrossRef] [Green Version]
- Hartung, T. Perspectives on in vitro to in vivo extrapolations. Appl. In Vitro Toxicol. 2018, 4, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Checkley, S.; MacCallum, L.; Yates, J.; Jasper, P.; Luo, H.; Tolsma, J.; Bendtsen, C. Bridging the gap between in vitro and in vivo: Dose and schedule predictions for the ATR inhibitor AZD6738. Sci. Rep. 2015, 5, 13545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paller, C.J.; Denmeade, S.R.; Carducci, M.A. Challenges of conducting clinical trials of natural products to combat cancer. Clin. Adv. Hematol. Oncol. 2016, 14, 447–455. [Google Scholar]
- Wu, H.; Chen, L.; Zhu, F.; Han, X.; Sun, L.; Chen, K. The cyto-toxicity effect of resvera-trol: Cell cycle arrest and induced apoptosis of breast cancer 4T1 cells. Toxins 2019, 11, 731. [Google Scholar] [CrossRef] [Green Version]
- Peng, L.; Jiang, D. Resveratrol eliminates cancer stem cells of osteosarcoma by STAT3 pathway inhibition. PLoS ONE 2018, 13, e0205918. [Google Scholar]
- Rodríguez-Enríquez, S.; Pacheco-Velázquez, S.C.; Marín-Hernández, Á.; Gallardo-Pérez, J.C.; Robledo-Cadena, D.X.; Hernández-Reséndiz, I.; García-García, J.D.; Belmont-Díaz, J.; López-Marure, R.; Hernández-Esquivel, L.; et al. Resveratrol inhibits cancer cell proliferation by impairing oxidative phosphorylation and inducing oxidative stress. Toxicol. Appl. Pharmacol. 2019, 370, 65–77. [Google Scholar] [CrossRef]
- Buhrmann, C.; Shayan, P.; Goel, A.; Shakibaei, M. Resveratrol regulates colorectal cancer cell invasion by modulation of focal adhesion molecules. Nutrients 2017, 9, 1073. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Han, X.; Zheng, S.; Li, Z.; Sha, Y.; Ni, J.; Sun, Z.; Qiao, S.; Song, Z. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol. Rep. 2016, 35, 1065–1074. [Google Scholar] [CrossRef] [Green Version]
- Lai, H.W.; Chien, S.Y.; Kuo, S.J.; Tseng, L.M.; Lin, H.Y.; Chi, C.W.; Chen, D.R. The potential utility of curcumin in the treatment of HER-2-overexpressed breast cancer: An in vitro and in vivo comparison study with herceptin. Evid. Based Complement Alternat Med. 2012, 2012, 486568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, H.; Chang, C.; Chou, Y.; Yeh, M.; Au, M.; Lu, H.; Chu, Y.; Chou, H.; Chou, H.; Shih, Y.; et al. Curcumin causes DNA damage and affects associated protein expression in HeLa human cervical cancer cells. Oncol. Rep. 2016, 36, 2207–2215. [Google Scholar] [CrossRef]
- Tong, W.; Wang, Q.; Sun, D.; Suo, J. Curcumin suppresses colon cancer cell invasion via AMPK-induced inhibition of NF-κB, uPA activator and MMP9. Oncol Lett. 2016, 12, 4139–4146. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, X.Q.; Zhang, Q.; Zhu, J.Y.; Li, Y.; Xie, C.F.; Li, X.T.; Wu, J.S.; Geng, S.S.; Zhong, C.Y.; et al. (−)-Epigallocatechin-3-Gallate inhibits colorectal cancer stem cells by suppressing Wnt/β-catenin pathway. Nutrients 2017, 9, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonoda, J.; Ikeda, R.; Baba, Y.; Narumi, K.; Kawachi, A.; Tomishige, E.; Nishihara, K.; Takeda, Y.; Yamada, K.; Sato, K.; et al. Green tea catechin, epigallocatechin-3-gallate, attenuates the cell viability of human non-small-cell lung cancer A549 cells via reducing Bcl-xL expression. Exp. Ther. Med. 2014, 8, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Hong, O.; Noh, E.; Jang, H.; Lee, Y.; Lee, B.; Jung, S.; Kim, J.; Youn, H. Epigallocatechin gallate inhibits the growth of MDA-MB-231 breast cancer cells via inactivation of the β-catenin signaling pathway. Oncol. Lett. 2017, 14, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.A.; Zhang, S.; Yin, Q.; Zhang, J. Quercetin induces human colon cancer cells apoptosis by inhibiting the nuclear factor-kappa B Pathway. Pharmacogn. Mag. 2015, 11, 404–409. [Google Scholar] [CrossRef] [Green Version]
- Guon, T.E.; Chung, H.S. Hyperoside and rutin of Nelumbo nucifera induce mitochondrial apoptosis through a caspase-dependent mechanism in HT-29 human colon cancer cells. Oncol. Lett. 2016, 11, 2463–2470. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Lee, S.R.; Kang, K.S.; Ko, Y.; Pang, C.; Yamabe, N.; Kim, K.H. Betulinic acid suppresses ovarian cancer cell proliferation through induction of apoptosis. Biomolecules 2019, 9, 257. [Google Scholar] [CrossRef] [Green Version]
- Zeng, A.; Hua, H.; Liu, L.; Zhao, J. Betulinic acid induces apoptosis and inhibits metastasis of human colorectal cancer cells in vitro and in vivo. Bioorg. Med. Chem. 2019, 27, 2546–2552. [Google Scholar] [CrossRef]
- Mondal, A.; Chatterji, U. Artemisinin represses telomerase subunits and induces apoptosis in HPV-39 infected human cervical cancer cells. J. Cell Biochem. 2015, 116, 1968–1981. [Google Scholar] [CrossRef]
- Garvin, S.; Ollinger, K.; Dabrosin, C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006, 231, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Monteillier, A.; Voisin, A.; Furrer, P.; Allémann, E.; Cuendet, M. Intranasal administration of resveratrol successfully prevents lung cancer in A/J mice. Sci Rep. 2018, 8, 14257. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Shin, H.; Kim, J. In vivo anti-cancer effects of resveratrol mediated by NK cell activation. J. Innate. Immun. 2020, 1–13. [Google Scholar] [CrossRef]
- Liu, A.; Zheng, R.; Yang, F.; Huang, L.; Zhang, L.; Zhang, J. Effects of curcumin on growth of human cervical cancer xenograft in nude mice and underlying mechanism. Food Sci. Technol. Campinas. 2017, 38, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, A.P.; Banerjee, S.; Nautiyal, J.; Patel, B.B.; Patel, V.; Du, J.; Yu, Y.; Elliott, A.A.; Levi, E.; Sarkar, F.H. Curcumin synergizes with resveratrol to inhibit colon cancer. Nutr. Cancer 2009, 61, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Gong, W.; Zhang, C.; Wang, S. Epigallocatechin gallate inhibits the proliferation of colorectal cancer cells by regulating Notch signaling. Onco. Targets Ther. 2013, 6, 145–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, Y.; Terashita, N.; Muraguchi, T.; Fukusato, T.; Kubota, S. Effects of epigallocatechin-3-gallate (EGCG) on A549 lung cancer tumor growth and angiogenesis. Biosc.i Biotechnol. Biochem. 2013, 77, 1799–1803. [Google Scholar] [CrossRef] [Green Version]
- Zan, L.; Chen, Q.; Zhang, L.; Li, X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered 2019, 10, 374–382. [Google Scholar] [CrossRef] [Green Version]
- Angst, E.; Park, J.L.; Moro, A.; Lu, Q.Y.; Lu, X.; Li, G.; King, J.; Chen, M.; Reber, H.A.; Go, V.L.; et al. The flavonoid quercetin inhibits pancreatic cancer growth in vitro and in vivo. Pancreas 2013, 42, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Castro, A.J.; Domínguez, F.; García-Carrancá, A. Rutin exerts antitumor effects on nude mice bearing SW480 tumor. Arch Med Res. 2013, 44, 346–351. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, Y.H.; Gao, L.W.; Li, X.Y.; Jin, Q.X.; Wang, Y.Y.; Xu, Y.Y.; Jin, F.; Lu, S.L.; Wei, M.J. Artemisinin enhances the anti-tumor immune response in 4T1 breast cancer cells in vitro and in vivo. Int. Immunopharmacol. 2019, 70, 110–116. [Google Scholar] [CrossRef]
- Li, L.N.; Zhang, H.D.; Yuan, S.J.; Tian, Z.Y.; Wang, L.; Sun, Z.X. Artesunate attenuates the growth of human colorectal carcinoma and inhibits hyperactive Wnt/beta-catenin pathway. Int. J. Cancer 2007, 121, 1360–1365. [Google Scholar] [CrossRef]
- Luo, J.; Zhu, W.; Tang, Y.; Cao, H.; Zhou, Y.; Ji, R.; Zhou, X.; Lu, Z.; Yang, H.; Zhang, S.; et al. Artemisinin derivative artesunate induces radiosensitivity in cervical cancer cells in vitro and in vivo. Radiat. Oncol. 2014, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Wong, V.K.; Cheung, S.S.; Li, T.; Jiang, Z.H.; Wang, J.R.; Dong, H.; Yi, X.Q.; Zhou, H.; Liu, L. Asian ginseng extract inhibits in vitro and in vivo growth of mouse lewis lung carcinoma via modulation of ERK-p53 and NF-κB signaling. J. Cell Biochem. 2010, 111, 899–910. [Google Scholar] [CrossRef]
- Pan, M.H.; Chiou, Y.S.; Chen, L.H.; Ho, C.T. Breast cancer chemoprevention by dietary natural phenolic compounds: Specific epigenetic related molecular targets. Mol. Nutr. Food Res. 2015, 59, 21–35. [Google Scholar] [CrossRef]
- Koh, Y.C.; Ho, C.T.; Pan, M.H. Recent advances in cancer chemoprevention with phytochemicals. J. Food Drug Anal. 2020, 28, 14–37. [Google Scholar] [CrossRef]
- Kiokias, S.; Proestos, C.; Oreopoulou, V. Effect of natural food antioxidants against LDL and DNA oxidative changes. Antioxidants 2018, 7, 133. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.M.; Chen, H.H.; Lin, C.A.; Wu, H.C.; Sheu, J.J.; Chen, H.J. Apigenin-induced lysosomal degradation of β-catenin in Wnt/β-catenin signaling. Sci Rep. 2017, 7, 372. [Google Scholar] [CrossRef]
- Narwal, M.; Haikarainen, T.; Fallarero, A.; Vuorela, P.M.; Lehtiö, L. Screening and structural analysis of flavones inhibiting tankyrases. J. Med. Chem. 2013, 56, 3507–3517. [Google Scholar] [CrossRef]
- Król, S.K.; Kiełbus, M.; Rivero-Müller, A.; Stepulak, A. Comprehensive review on betulin as a potent anticancer agent. Biomed. Res. Int. 2015, 2015, 584189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghante, M.H.; Jamkhande, P.G. Role of pentacyclic triterpenoids in chemoprevention and anticancer treatment: An overview on targets and underling mechanisms. J. Pharmacopuncture. 2019, 22, 55–67. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A. Betulin and betulinic acid in cancer research. J. Pre. Clin. Clin. Res. 2018, 12, 72–75. [Google Scholar] [CrossRef]
- Danciu, C.; Pinzaru, I.; Coricovac, D.; Andrica, F.; Sizemore, I.; Dehelean, C.; Baderca, F.; Lazureanu, V.; Soica, C.; Mioc, M.; et al. Betulin silver nanoparticles qualify as efficient antimelanoma agents in in vitro and in vivo studies. Eur. J. Pharm. Biopharm. 2019, 134, 1–19. [Google Scholar] [CrossRef]
- Chen, H.; Xiao, H.; Pang, J. Parameter optimization and potential bioactivity evaluation of a betulin extract from white birch bark. Plants 2020, 9, 392. [Google Scholar] [CrossRef] [Green Version]
- Pfarr, K.; Danciu, C.; Arlt, O.; Neske, C.; Dehelean, C.; Pfeilschifter, J.M.; Radeke, H.H. Simultaneous and dose dependent melanoma cytotoxic and immune stimulatory ac-tivity of betulin. PLoS ONE 2015, 10, e0118802. [Google Scholar] [CrossRef]
- De Oliveira Júnior, R.G.; Christiane Adrielly, A.F.; da Silva Almeida, J.R.G.; Grougnet, R.; Thiéry, V.; Picot, L. Sensitization of tumor cells to chemotherapy by natural products: A systematic review of preclinical data and molecular mechanisms. Fitoterapia 2018, 129, 383–400. [Google Scholar] [CrossRef] [PubMed]
- Herranz-López, M.; Losada-Echeberría, M.; Barrajón-Catalán, E. The multitarget activity of natural extracts on cancer: Synergy and xenohormesis. Medicines 2018, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.C.; Kannappan, R.; Reuter, S.; Kim, J.H.; Aggarwal, B.B. Chemosensitization of tumors by resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 150–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Seedi, H.R.; Yosri, N.; Khalifa, S.A.M.; Guo, Z.; Musharraf, S.G.; Xiao, J.; Saeed, A.; Du, M.; Khatib, A.; Abdel-Daim, M.M.; et al. Exploring natural products-based cancer therapeutics derived from egyptian flora. J. Ethnopharmacol. 2021, 269, 113626. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guestini, F.; McNamara, K.M.; Sasano, H. The use of chemosensitizers to enhance the response to conventional therapy in triple-negative breast cancer patients. Breast Cancer Manag. 2018, 6, 127–131. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Li, W.; Wang, R.; Nan, Y.; Wang, Q.; Liu, W.; Jin, F. Resveratrol enhanced anticancer effects of cisplatin on non-small cell lung cancer cell lines by inducing mitochondrial dysfunction and cell apoptosis. Int. J. Oncol. 2015, 47, 1460–1468. [Google Scholar] [CrossRef] [Green Version]
- Carlson, L.J.; Cote, B.; Alani, A.W.; Rao, D.A. Polymeric micellar co-delivery of resveratrol and curcumin to mitigate in vitro doxorubicin-induced cardiotoxicity. J. Pharm. Sci. 2014, 103, 2315–2322. [Google Scholar] [CrossRef]
- Cote, B.; Carlson, L.J.; Rao, D.A.; Alani, A.W.G. Combinatorial resveratrol and quercetin polymeric micelles mitigate doxorubicin induced cardiotoxicity in vitro and in vivo. J. Control. Release. 2015, 213, 128–133. [Google Scholar] [CrossRef]
- Kang, Y.; Hu, W.; Bai, E.; Zheng, H.; Liu, Z.; Wu, J.; Jin, R.; Zhao, C.; Liang, G. Curcumin sensitizes human gastric cancer cells to 5-fluorouracil through inhibition of the NF-κB survival-signaling pathway. Onco. Targets Ther. 2016, 9, 7373–7384. [Google Scholar] [CrossRef] [Green Version]
- Sreekanth, C.N.; Bava, S.V.; Sreekumar, E.; Anto, R.J. Molecular evidences for the chemosensitizing efficacy of liposomal curcumin in paclitaxel chemotherapy in mouse models of cervical cancer. Oncogene 2011, 30, 3139–3152. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, R.; Kang, Y.; Li, X.; Roife, D.; Zhang, R.; Fleming, J.B. Genistein potentiates the antitumor effect of 5-Fluorouracil by inducing apoptosis and autophagy in human pancreatic cancer cells. Anticancer Res. 2014, 34, 4685–4692. [Google Scholar] [PubMed]
- Puglia, C.; Lauro, M.R.; Tirendi, G.G.; Fassari, G.E.; Carbone, C.; Bonina, F.; Puglisi, G. Modern drug delivery strategies applied to natural active compounds. Expert Opin. Drug Deliv. 2017, 14, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.A.; Al Qaraghuli, M.M.; Alsaadi, M.; Alzahrani, A.R.; Niwasabutra, K.; Ferro, V.A. Delivering natural products and biotherapeutics to improve drug efficacy. Ther. Deliv. 2017, 8, 947–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Ahmed, B.; Mehta, K.; Kurzrock, R. Liposomal curcumin with and without oxali-platin: Effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol. Cancer Ther. 2007, 6, 1276–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.E.; Ngai, S.C.; Chan, K.G.; Lee, L.H.; Goh, B.H.; Chuah, L.H. Curcumin nanoformulations for colorectal cancer: A review. Front Pharmacol. 2019, 10, 152. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Cao, Y.N.; Sun, J.; Liang, Z.; Wu, Q.; Cui, S.H.; Zhi, D.F.; Guo, S.T.; Zhen, Y.H.; Zhang, S.B. Anti-breast cancer activity of resveratrol encapsulated in liposomes. J. Mater. Chem. B 2020, 8, 27–37. [Google Scholar] [CrossRef]
- Sanna, V.; Singh, C.K.; Jashari, R.; Adhami, V.M.; Chamcheu, J.C.; Rady, I.; Sechi, M.; Mukhtar, H.; Siddiqui, I.A. Targeted nanoparticles encapsulating (−)-epigallocatechin-3-gallate for prostate cancer prevention and therapy. Sci. Rep. 2017, 7, 41573. [Google Scholar] [CrossRef] [Green Version]
- Rastegar, R.; Akbari Javar, H.; Khoobi, M.; Dehghan Kelishadi, P.; Hossein Yousefi, G.; Doosti, M.; Hossien Ghahremani, M.; Shariftabrizi, A.; Imanparast, F.; Gholibeglu, E.; et al. Evaluation of a novel biocompatible magnetic nanomedicine based on beta-cyclodextrin, loaded doxorubicin-curcumin for overcoming chemoresistance in breast cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Yang, Y.; Liu, Y.; Pan, J.; Wang, J.; Man, F.; Zhang, W.; Liu, G. Melanin-like nanomaterials for advanced biomedical applications: A versatile platform with extraordinary promise. Adv. Sci. 2020, 7, 1903129. [Google Scholar] [CrossRef] [Green Version]
- Caldas, M.; Santos, A.C.; Veiga, F.; Rebelo, R.; Reis, R.L.; Correlo, V.M. Melanin nanoparticles as a promising tool for biomedical applications-A review. Acta Biomater. 2020, 105, 26–43. [Google Scholar] [CrossRef]
- Ozlu, B.; Kabay, G.; Bocek, I.; Yilmaz, M.; Piskin, A.K.; Shim, B.S.; Mutlu, M. Controlled release of doxorubicin from polyethylene glycol functionalized melanin nanoparticles for breast cancer therapy: Part I. Production and drug release performance of the melanin nanoparticles. Int. J. Pharm. 2019, 570, 118613. [Google Scholar] [CrossRef]
- Wang, K.; Wang, S.; Chen, K.; Zhao, Y.; Ma, X.; Wang, L. Doxorubicin-loaded melanin particles for enhanced chemotherapy in drug-resistant anaplastic thyroid cancer cells. J. Nanomater. 2018, 2018, 2603712. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Cai, L.; Tian, Z.; Wu, Y.; Chen, J. Phytochemical curcumin-coformulated, silver-decorated melanin-like polydopamine/mesoporous silica composites with improved antibacterial and chemotherapeutic effects against drug-resistant cancer cells. ACS Omega 2020, 5, 15083–15094. [Google Scholar] [CrossRef]
- Mioc, M.; Pavel, I.Z.; Ghiulai, R.; Coricovac, D.E.; Farcaş, C.; Mihali, C.V.; Oprean, C.; Serafim, V.; Popovici, R.A.; Dehelean, C.A.; et al. The cytotoxic effects of betulin-conjugated gold nanoparticles as stable formula-tions in normal and melanoma cells. Front Pharmacol. 2018, 9, 429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falamas, A.; Dehelean, C.; Cinta Pinzaru, S. Monitoring of betulin nanoemulsion treatment and molecular changes in mouse skin cancer using surface enhanced Raman spectroscopy. Vib. Spectrosc. 2018, 95, 44–50. [Google Scholar] [CrossRef]
- Farcas, C.G.; Dehelean, C.; Pinzaru, I.A.; Mioc, M.; Socoliuc, V.; Moaca, E.A.; Avram, S.; Ghiulai, R.; Coricovac, D.; Pavel, I.; et al. Ther-mosensitive betulinic acid-loaded magnetoliposomes: A promising antitumor poten-tial for highly aggressive human breast adenocarcinoma cells under hyperthermic conditions. Int. J. Nanomed. 2020, 15, 8175–8200. [Google Scholar] [CrossRef]
- Wang, X.; Parvathaneni, V.; Shukla, S.K.; Kanabar, D.D.; Muth, A.; Gupta, V. Cyclodextrin complexation for enhanced stability and non-invasive pulmonary delivery of resveratrol-applications in non-small cell lung cancer treatment. AAPS PharmSciTech. 2020, 21, 183. [Google Scholar] [CrossRef] [PubMed]
- Thipe, V.C.; Panjtan Amiri, K.; Bloebaum, P.; Raphael Karikachery, A.; Khoobchandani, M.; Katti, K.K.; Jurisson, S.S.; Katti, K.V. Development of resveratrol-conjugated gold nanoparticles: Interrelationship of increased resveratrol corona on anti-tumor efficacy against breast, pancreatic and prostate cancers. Int. J. Nanomed. 2019, 14, 4413–4428. [Google Scholar] [CrossRef] [Green Version]
- Ranjan, A.P.; Mukerjee, A.; Helson, L.; Gupta, R.; Vishwanatha, J.K. Efficacy of liposomal curcumin in a human pancreatic tumor xenograft model: Inhibition of tumor growth and angiogenesis. Anticancer Res. 2013, 33, 3603–3609. [Google Scholar]
- Zhang, L.; Man, S.; Qiu, H.; Liu, Z.; Zhang, M.; Ma, L.; Gao, W. Curcumin-cyclodextrin complexes enhanced the anti-cancer effects of curcumin. Environ. Toxicol. Pharmacol. 2016, 48, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Marwah, M.; Perrie, Y.; Badhan, R.K.S.; Lowry, D. Intracellular uptake of EGCG-loaded deformable controlled release liposomes for skin cancer. J. Liposome. Res. 2020, 30, 136–149. [Google Scholar] [CrossRef]
- Zeng, L.; Yan, J.; Luo, L.; Ma, M.; Zhu, H. Preparation and characterization of (−)-Epigallocatechin-3-gallate (EGCG)-loaded nanoparticles and their inhibitory effects on Human breast cancer MCF-7 cells. Sci Rep. 2017, 28, 45521. [Google Scholar] [CrossRef]
- Niazvand, F.; Orazizadeh, M.; Khorsandi, L.; Abbaspour, M.; Mansouri, E.; Khodadadi, A. Effects of quercetin-loaded nanoparticles on MCF-7 human breast cancer cells. Medicina. 2019, 55, 114. [Google Scholar] [CrossRef] [Green Version]
- Baksi, R.; Singh, D.P.; Borse, S.P.; Rana, R.; Sharma, V.; Nivsarkar, M. In vitro and in vivo anticancer efficacy potential of Quercetin loaded polymeric nanoparticles. Biomed. Pharmacother. 2018, 106, 1513–1526. [Google Scholar] [CrossRef]
- Pinzaru, I.; Tanase, A.; Enatescu, V.; Coricovac, D.; Bociort, F.; Marcovici, I.; Watz, C.; Vlaia, L.; Soica, C.; Dehelean, C. Proniosomal gel for topical delivery of rutin: Preparation, physicochemical characterization and in vitro toxicological profile using 3D reconstructed human epidermis tissue and 2D cells. Antioxidants 2021, 10, 85. [Google Scholar] [CrossRef]
- Danciu, C.; Bojin, F.; Pinzaru, I.; Dehelean, C.; Ambrus, R.; Popescu, A.; Paunescu, V.; Hancianu, M.; Minda, D.; Soica, C. Rutin and its cyclodextrin inclusion complexes: Physico-chemical evaluation and in vitro activity on B164A5 murine melanoma cell line. Curr. Pharm. Biotechnol. 2017, 18, 1067–1077. [Google Scholar] [CrossRef]
- Pinzaru, I.; Sarau, C.; Coricovac, D.; Marcovici, I.; Utescu, C.; Tofan, S.; Popovici, R.A.; Manea, H.C.; Pavel, I.E.; Soica, C.; et al. Silver nanocolloids loaded with betulinic acid with enhanced antitumor potential: Physicochemical characterization and in vitro evaluation. Nanomaterials 2021, 11, 152. [Google Scholar] [CrossRef]
- Natesan, S.; Ponnusamy, C.; Sugumaran, A.; Chelladurai, S.; Shanmugam Palaniappan, S.; Palanichamy, R. Artemisinin loaded chitosan magnetic nanoparticles for the efficient targeting to the breast cancer. Int. J. Biol. Macromol. 2017, 104, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gallis, B.; Taya, M.; Wang, S.; Ho, R.J.; Sasaki, T. pH-responsive artemisinin derivatives and lipid nanoparticle formulations inhibit growth of breast cancer cells in vitro and induce down-regulation of HER family members. PLoS ONE 2013, 8, e59086. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Zhu, W.; Si, C.; Lei, J. "Nano-Ginseng" for enhanced cytotoxicity against cancer cells. Int. J. Mol. Sci. 2018, 19, 627. [Google Scholar] [CrossRef] [Green Version]
- Hong, C.; Wang, D.; Liang, J.; Guo, Y.; Zhu, Y.; Xia, J.; Qin, J.; Zhan, H.; Wang, J. Novel ginsenoside-based multifunctional liposomal delivery system for combination therapy of gastric cancer. Theranostics 2019, 9, 4437–4449. [Google Scholar] [CrossRef]








| Natural Compound | Cancer Type | Cell Line (s) | Active Concentrations | Findings | Cell Death Type | Ref. |
|---|---|---|---|---|---|---|
| Resveratrol | Breast cancer | 4T1 | IC50 = 93 µM (72 h) | Cell cycle inhibition; S-phase arrest; ↑ cell apoptosis rate | Apoptosis | [60] |
| Osteosarcoma | MNNG/HOS | IC50 = 20.57 μM (48 h) | Cleavage of PARP, and caspase-3; ↑ Bax; ↓ Bcl-2 and Bcl-xL; JAK2/STAT3 pathway inhibition | Apoptosis | [61] | |
| MG-63 | IC50 = 28.56 μM (48 h) | |||||
| Cervical cancer | HeLa | IC50 = 188 ± 19 µM (48 h) | ↑ content of LAMP1, Atg7, LC3B, PINK1 and PARK2 proteins; ROS overload; ↓ glycolysis; ↓ oxidative phosphorylation protein contents and fluxes | Autophagy | [62] | |
| Colorectal cancer | SW480 | 5 µM | inducing cell cluster formation; ↓ cell viability | Apoptosis | [63] | |
| HCT116 | ||||||
| Curcumin | Melanoma | A375 | IC50 = 25 µM (24 h) | inhibition of cell invasion; G2/M phase cell-cycle arrest; suppression of the AKT, mTOR and P70S6K activation | Autophagy | [64] |
| C8161 | IC50 = 15 µM (24 h) | |||||
| Breast Cancer | MCF-7 | 1–25 μg/mL (72 h) | ↓ phosphorylation of Akt and MAPK; ↓ HER-2 oncoprotein; ↓ NF-κB | - | [65] | |
| MDA-MB-231 | ||||||
| BT-474 | ||||||
| SK-BR-3-hr | ||||||
| Cervical Cancer | HeLa | 12–14 µM (48 h) | DNA damage and fragmentation; chromatin condensation; ↑ amounts of p-ATM, p-ATR, p53, MDM2, BRCA1, DNA-PK, MDC1 and p-H2A.X, PARP and MGMT proteins | Apoptosis | [66] | |
| Colon Cancer | SW480 | 1–50 µM (24 h) | inhibition of the cellular invasive activity; ↓ uPA and MMP9 expression; ↓ NF-κB activation | - | [67] | |
| LoVo | ||||||
| EGCG | Colorectal Cancer | DLD-1 | 20–60 µM | ↓ number and size of cell sphe-roids;inhibition of the Wnt/β-catenin pathway; ↓ pro-tein levels of Cyclin D1 and PCNA; ↓ Bcl-2; ↑ Bax, Caspase-8, Caspase-9, and Caspase-3 | Apoptosis | [68] |
| SW480 | ||||||
| Lung Cancer | A549 | IC50 = 36.0 μM (72 h) | ↓ survival rate; loss of the adhe-sion ability; ↓ Bcl-xL | Apoptosis | [69] | |
| Breast Cancer | MDA-MB-231 | ≥ 75 μM (24 h) | ↓ expression of β-catenin, p-Akt, and cyclin D1; inactivation of the β catenin signaling pathway; ↓ cell proliferation; disrupted adherence junction formation | - | [70] | |
| Quercetin | Pancreatic Cancer | LNCaP DU-145 PC-3 | 40 μM (24, 48, and 72 h) | modulation of ROS production and mitochondrial membrane potential; interference with MAPK, Akt, and NF-κB signaling pathways | Apoptosis Necrosis | [38] |
| Cervical Cancer | HeLa | EC50 = 100 μM (24 h) | ↑ expression of caspases and pro-apoptotic genes; DNA fragmentation; ↓ cell migration; G2-M cell cycle arrest; blockage of the PI3K, MAPK and WNT pathways | Apoptosis | [37] | |
| Colorectal Adenocarcinoma | Caco-2 | IC50 = 35 μM (24 h) | NF-κB pathway inhibition; ↑Bax; ↓Bcl-2 increased cell membrane permeability; nuclear condensation | Apoptosis | [71] | |
| Rutin | Colon Cancer | HT-29 | 100 and 200 μM (24 h) | ↑ cleaved caspases-3, -8 and -9; ↓ Bcl 2; ↑ Bax; cell shrinkage; chromatin condensation; rounding, blebbing and an increased density of apoptotic bodies | Apoptosis | [72] |
| Betulinic acid | Melanoma | B164A5 | 10 mM | G0/G1 cell cycle arrest; ↓ cell proliferation | Apoptosis | [46] |
| Ovarian Cancer | A2780 | IC50 = 44.47 μM (24 h) | ↑ levels of cleaved caspase-8, -3, -9; ↑ Bax; ↓ Bcl-2; nuclear Condensation | Apoptosis | [73] | |
| Colon Cancer | HCT116 SW480 DLD-1 | ≥ 5 µg/mL (48 and 72 h) | ↑ Bax; ↑ cleaved caspase-3; ↑ ROS production; ↓ Bcl-2; ↓ mitochondrial membrane potential | Apoptosis | [74] | |
| Artemisinin (Artesunate) | Breast Cancer | MDA-MB-231 | 25, 50 and 100 μM (48 h) | ↓ Bcl-2; ↑ Bax; G2/M-phase arrest; ↓ Cyclin-B1 and Cyclin-D1; agglutinated heterochromatin; degenerated mitochondrial vacuoles; nuclear swelling; ↓ number of intracellular organelles | Apoptosis Autophagy | [50] |
| Colon Cancer | HCT116 | 40 and 80 μM (24, 48, and 72 h) | Cell elongation; membrane foaming; nuclear condensation and fragmentation; chromatin shrinkage; ↓ Bcl 2 and Bcl xL; ↑ Bax; ↑ cleaved procaspase-3 to active caspase-3; ↑ levels of beclin 1, LC3 I/II, and Atg5; ↑ complexation of Atg12-Atg5 | Apoptosis Autophagy | [51] | |
| Cervical Cancer | ME-180 | 300 and 500 μM (48 and 72 h) | ↓ Telomerase activity; ↓ hTERT and hTR expression; ↓ E6 and E7 oncogenes; chromatin condensation; ↑ p53 expression | Apoptosis | [75] | |
| Ginseng extract | Breast Cancer | MCF-7 | 100–400 μM (24 h) | ↓ Bcl-2; ↑ Bax, cytochrome c, and cleaved caspase-3; ↑ ROS production | Apoptosis | [55] |
| Lung Cancer | A549 | 50–100 μM (48 and 72 h) | Punctate cytoplasmic expression of LC3, Beclin-1 and ATG5; G2/M phase arrest; ↑ expression of LC3-II; ↑ p-Akt; ↓ mTOR-4EBP1 | Autophagy | [56] |
| Natural Compound | Drug Delivery System | Findings | Ref. |
|---|---|---|---|
| Resveratrol | Peptide and Sucrose Liposomes | Prolonged drug-release in vitro; Breast cancer (MCF-7) cells growth inhibition (IC50 = 20.89 μmol/L); Apoptosis; Tumor growth inhibition in mice bearing breast cancer (Dose = 10 mg/kg) | [128] |
| Sulfobutylether-β-cyclodextrin | Improved stability; Longer half-life; Significant cytotoxic potential against non-small cell lung cancer; Suitable for pulmonary delivery | [117] | |
| Gold Nanoparticles | Optimal cellular uptake; Superior cytotoxic effects on breast, pancreatic, and prostate cancers | [129] | |
| Curcumin | Silver-Decorated Melanin-like Polydopamine/Mesoporous Silica Composites | Improved chemotherapeutic efficiency against human cervical cancer cells (HeLa) and Taxol-resistant non-small cell lung cells (A549/TAX); Desirable biocompatibility; Low hemolytic activity | [124] |
| Liposomes | Potent cytotoxic effect against human MiaPaCa pancreatic cancer cells (IC50 = 17.5 µM); suppression of tumor growth in tumor-bearing nude mice (Dose = 20 mg/kg); Potent antiangiogenic effect | [130] | |
| Cyclodextrins | Enhanced delivery; Improved therapeutic efficacy against lung cancer in vitro and in vivo | [131] | |
| EGCG | Deformable Liposomal Formulation | Increased drug-release; Optimal formulation for topical delivery in skin cancer prevention | [132] |
| Folic acid and polyethylene glycol (PEG)-modified Nanoparticles | Inhibition of MCF-7 cells proliferation in a dose-dependent manner; Enhances targeting ability and efficacy of the drug | [133] | |
| Quercetin | Lipid Nanoparticles | Sustained release; Inhibition of the MCF-7 breast cancer cells growth; Increased ROS production; Increased apoptotic and necrotic indexes in MCF-7 cells | [134] |
| Polymeric Nanoparticles | Enhanced efficacy in cancer therapy; Reduced tumor volume in breast and lung-bearing mice; | [135] | |
| Rutin | Proniosomal Gel | High biocompatibility of the gel on the 3D reconstructed human epidermis; Lack of irritant and phototoxic potential; Preferential cytotoxic effect of the drug on melanoma cells (IC50 = 8.601 µM) | [136] |
| β-cyclodextrins and hydroxypropyl-β-cyclodextrins | Increased antioxidant activity; Antiproliferative and pro-apoptotic effect against B164A5 murine melanoma cells | [137] | |
| Betulinic acid | Gamma-cyclodextrins | Improved antiproliferative activity in vitro on metastatic and non-metastatic B164A5 melanoma cells; G0/G1 cell-cycle arrest; Reduced in vivo tumor development | [46] |
| Magnetoliposomes | Enhanced antitumor activity when breast adenocarcinoma MDA-MB-231 cells and a microtubule assembly modulatory activity under hypertermic conditions; | [127] | |
| Silver Nanocolloids | Augmented anticancer effect against lung A549 and liver HepG2 cancer cell lines; Cell type- and time-dependent cytotoxic effect | [138] | |
| Artemisinin (Artesunate) | Chitosan Magnetic Nanoparticles | Enhanced accumulation of nanoparticles in the 4T1 breast tumor tissues of BALB/c mice model | [139] |
| pH-Responsive Lipid Nanoparticles | Inhibition of the breast cancer cells growth; down-regulation of the anti-apoptotic protein survivin, and cyclin D1; down-regulation of the oncogenic proteins HER2 and HER3; Reduced expression of the epidermal growth factor receptor (EGFR or HER1) | [140] | |
| Ginseng | Ginsenoside Rb1/Protopanaxadiol Nanoparticles | High encapsulation efficiency, drug loading capacity, and slow release kinetics; Lack of hemolytic effect; Superior in vitro anticancer activity on murine Lewis lung carcinoma | [141] |
| Ginsenoside-based multifunctional liposomal delivery system | Successful delivery of the bioactive combination drugs and internalization into gastric cancer cells; Suppressed gastric cancer tumor growth | [142] |
| Natural Compound | Cancer Type | Animal Model | Dose/Administration | Findings | Ref. |
|---|---|---|---|---|---|
| Resveratrol | Breast cancer | MDA-MB-231 cells xenograft model in female athymic mice | intraperitoneal administration of 25 mg/kg/day of resveratrol (ethanolic solution) for 3 weeks | ↓ tumor size; ↑ apoptotic index; ↓ angiogenesis | [76] |
| Lung cancer | A/J mice with 4-[methyl(nitroso)amino]-1-(3-pyridinyl)-1-butanone-induced lung carcinogenesis | intranasal administration of ~60 mg/kg three times a week for 25 weeks | ↓ tumor size (27%); ↓ tumor volume (45%) chemopreventive properties in the chemically-induced lung cancer | [77] | |
| Melanoma | B16F10 cells xenograft model in female C57BL/6 mice | intravenous administration of 0.5 mg/kg of resveratrol (PBS solution) 6 times on days −2, 0, 2, 4, 6, and 8 | inhibition of tumor growth and metastasis; ↑ NK cell activity | [78] | |
| Curcumin | Breast Cancer | HER-2-overexpressed BT-474 xenograft mod-el in female athymic nude mice | intraperitoneal administration of curcumin dissolved in 0.1% DMSO at a dose of 45 mg/kg twice/week for 4 consecutive weeks | ↓ tumor volume (by 76.7%) | [65] |
| Cervical Cancer | Human cervical cancer HeLa cells xenograft model in nude mice | intraperitoneal administration of curcumin at a dosage of 50, 100 and 200 mg/kg, once/two days for 20 days | ↓ tumor volume and mass; tumor inhibition rate percentages were 1.7%, 31.1% and 39.6% for curcumin 50, 100 and 200, respectively | [79] | |
| Colon Cancer | HCT-116 Cells Xeno-graft model in female homozygous ICR SCID mice | 500 mg/kg body by gavage every day for 3 weeks | ↓ growth of p53-positive (wt) and p53-negative colon cancer HCT-116 cells; ↓ proliferation and ↑ apoptosis accompanied by the attenuation of NF-κB activity; synergistic effect with resveratrol | [80] | |
| EGCG | Colorectal Cancer | Orthotopic colorectal cancer xenograft model in BALB/c nude mice | intragastrical administration of 5, 10 and 20 mg/kg of EGCG once daily for 14 days | ↓ tumor volume; ↑ apoptotic rates for EGCG 5, 10, and 20 mg/kg (38.04%, 51.87%, 52.27%, and 54.33%) | [81] |
| Lung Cancer | A549 cells xenograft model in BALB/c nude male mice | 0.05% EGCG solutions in DMSO administered in drinking water daily for 13–21 days | ↓ tumor growth; ↓ angiogenesis and CD34 positive vessels | [82] | |
| Breast Cancer | MCF-7 cells xenograft model in female CB-17 severe combined im-munodeficient mice | 100 mg/kg of EGCG dissolved in 100 μL water every 2 days by oral gavage | ↓ tumor growth; ↓ expression of miR-25; ↓ Ki-67 and ↑ pro-apoptotic PARP expression | [83] | |
| Quercetin | Pancreatic Cancer | MIA PaCa-2 cells Orthotopic xenograft model in nude mice | 1% quercetin -supplemented diet for 42 days (Oral administration) | ↓ tumor volume and weight; ↓ tumor cell proliferation; ↑ apoptotic cell death | [84] |
| Rutin | Colon Cancer | SW480 cells xenograft in nu/nu mice | daily intraperitoneal administration of rutin at different doses (≤ 20 mg/kg) for 32 days | ↓ tumor volume and weight; ↑ mean survival time by 50 days; ↓ VEGF serum levels (by 55%) | [85] |
| Betulinic acid | Colorectal cancer | HCT116 cells xenograft model in mice | daily intraperitoneal administration of 10 and 20mg/kg/day of betulinic acid for 21 days | ↓ tumor growth; ↓ number of Ki67-positive and MMP-2-positive cells; ↑ cleaved caspase-3-positive cells | [74] |
| Artemisinin (Artesunate) | Breast Cancer | 4T1 cells xenograft model in female BALB/c mice | intraperitoneal injection with 100 mg/kg artemisinin dissolved in a 0.2% DMSO solution) daily, for 20 days | ↓ Treg and MDSC expansion in the spleen and tumor; ↑ percentages of CD4 + IFN-γ + T cells; ↑ FN-γ and TNF-α | [86] |
| Colorectal Cancer | CLY cells xenograft model in female athymic nude mice (Balb/c nu/nu) | intravenous administration of artesunate as follows: (1) intermittent large dose treatment (300 mg/kg; every 3 days, for 7 days) and (2)persistent small dose treatment (100 mg/kg; every day, for 20 days) | slow growth of tumor xenografts; ↓ physiological activity of tumor xenografts; delayed spontaneous liver metastasis. | [87] | |
| Cervical Cancer | HeLa cells xenografts in male BALB/c mice | intraperitoneal administration of 100 mg/kg/day artesunate for 7 days | ↓ microvessel density; ↑ apoptosis; ↑ Cyclin B1 expression G2-M phase arrest; ↑ radio-sensitivity | [88] | |
| Ginseng | Breast Cancer | MCF-7 cells xenograft model in female BALB/c athymic nude mice | intravenous administration of Ginseng extract (50 or 100 mg/kg), once a day for 4 weeks | ↓ tumor weight; ↑ Bax, cleaved caspase-3, and cleaved PARP; ↓ Bcl-2 | [55] |
| Lung Cancer | LLC-1 cells xenograft model in male C57BL/6J mice | Asian Ginseng extract (0.25, 0.5, and 1 g/kg/day) daily administration as pretreatment (for 10 days) and treatment (for 20 days) | ↓ tumor volume and mass; ↓ cell proliferative index; ↓ P-Stat3 and PCNA | [89] |
| Natural Compound | Identifier/Status | Cancer Type/Conditions | Title | Observations |
|---|---|---|---|---|
| Resveratrol | NCT00256334/Completed | Colon cancer | Resveratrol for Patients With Colon Cancer | Patients were randomly assigned to one of four dose cohorts: plant-derived resveratrol tablets (80 mg/day and 20 mg/day), Grape Powder dissolved in water and taken orally (120 g/day and 80 g/day). |
| NCT02261844/Withdrawn (No funding) | Liver cancer | Resveratrol and Human Hepatocyte Function in Cancer | Resveratrol 1 g daily for 10 days Dietary Supplement: Resveratrol Resveratrol 1 g po x 10 days prior to liver resection | |
| NCT01476592/Completed | Neuroendocrine tumor | A Biological Study of Resveratrol’s Effects on Notch-1 Signaling in Subjects With Low Grade Gastrointestinal Tumors | 5 g/day of resveratrol orally, in two divided doses of 2.5 g each without a break in therapy for a total of three cycles | |
| NCT00433576/Completed | Colorectal cancer | Resveratrol in Treating Patients With Colorectal Cancer That Can Be Removed By Surgery | STAGE II: Patients receive oral resveratrol on days 1–8 and undergo colorectomy on day 9. | |
| NCT00098969/Completed | Unspecified Adult Solid Tumor, Protocol Specific | UMCC 2003-064 Resveratrol in Preventing Cancer in Healthy Participants | This phase I trial is studying the side effects and best dose of resveratrol in preventing cancer in healthy participants. | |
| NCT03253913/Unknown | Lymphangioleiomyomatosis | Resveratrol and Sirolimus in Lymphangioleiomyomatosis Trial | Resveratrol 250 mg daily for the first 8 weeks, followed by 250 mg twice daily for the next 8 weeks, and then 500mg twice daily for the last 8 weeks. | |
| NCT04266353/Suspended (Due to COVID-19) | Breast cancer | Effect of Resveratrol on Serum IGF2 Among African American Women | Participants with receive resveratrol at 150 mg daily for 6 weeks | |
| NCT00578396/Unknown | Colon cancer | Phase I Biomarker Study of Dietary Grape-Derived Low Dose Resveratrol for Colon Cancer Prevention | - | |
| Curcumin | NCT03980509/Recruiting | Breast Cancer | A "Window Trial" on Curcumin, the Active Compound in Turmeric, for Invasive Breast Cancer Primary Tumors | Curcumin (500 mg) will be orally administered twice a day, after each meal from the time surgical resection is scheduled until the night before surgical resection. |
| NCT04294836/Not yet Recruiting | Cervical Cancer | Randomized Phase II Clinical Trial of Oral Turmeric Supplementation in Patients With Advanced Cervical Cancer | Curcumin administered in a dosage of 2000 mg daily, in association with cisplatin and radiotherapy for 16 weeks | |
| NCT02724202/Active, not recruiting | Colon Cancer | A Pilot, Feasibility Study of Curcumin in Combination With 5FU for Patients With 5FU-Resistant Metastatic Colon Cancer | Curcumin at a dosage of 500 mg twice/day will be orally administered for 2 weeks. Patients will continue on curcumin at the same dose for an additional 6 weeks while being treated with 3 cycles of 5-fluorouracil | |
| EGCG | NCT02891538/Recruiting | Colorectal Cancer | A Pilot Study to Evaluate the Chemopreventive Effects of Epigallocatechin Gallate (EGCG) in Colorectal Cancer (CRC) Patients With Curative Resections | EGCG (highly purified and refined green tea extract-Teavigo™) administered at a dosage of 450 mg twice a day |
| NCT01317953/Available | Lung Cancer | Phase I Study of Oral Green Tea Extract as Maintenance Therapy for Extensive-stage Small Cell Lung Cancer | Increasing doses of EGCG (400, 800, 1200, 1600 and 2000 mg) administered daily | |
| NCT00917735/Completed | Breast Cancer | Phase II, Randomized, Double-blind, Placebo-controlled, Study of the Efficacy of Green Tea Extract on Biomarkers of Breast Cancer Risk in High Risk Women With Differing Catechol-O-methyl Transferase (COMT) Genotypes | Oral administration of two Green tea extract capsules containing 51.7% EGCG, twice daily after breakfast and dinner for one year. | |
| Quercetin | NCT01538316/ Unknown | Prostate Cancer | Clinical Trial on the Effectiveness of the Flavonoids Genistein and Quercetin in Men With Rising Prostate-specific Antigen | 500 mg of quercetin supplement (+ vitamin C + folic acid + vitamin B3) administered daily over a period of six months, followed by genistein and placebo administration. |
| NCT03476330/Recruiting | Squamous Cell Carcinoma | Quercetin Chemoprevention for Squamous Cell Carcinoma in Patients With Fanconi Anemia | Quercetin administered orally twice daily at an wheight-based adjusted dosage (maximum 4000 mg/day). | |
| Betulinic acid | NCT00346502/Suspended | Dysplastic Nevus Syndrome | Phase I/II Evaluation of Topical Application of 20% Betulinic Acid Ointment in the Treatment of Dysplastic Nevi With Moderate to Severe Dysplasia | Daily application of the 20% betulinic acid ointment to the dysplastic nevi site for a period of four weeks. |
| Artemisimin (Artesunate) | NCT00764036/Completed | Breast Cancer | Prospective Open Uncontrolled Phase I Study of Compatibility, Safety&Pharmacokinetics of Artesunate, a Semisynthetic Derivative of Artemisinin From the Chinese Herb Artemisia Annua in Patients With Metastatic/Locally Advanced Breast Cancer | The administration of the drug was as follows: daily single oral doses of 100, 150 or 200 mg of artesunate, for 4 weeks. |
| NCT03093129/Recruiting | Colorectal Cancer | Phase II Randomised, Double Blind, Placebo Controlled Trial of Neoadjuvant Artesunate in Stage II/III Colorectal Cancer in Vietnamese Patients | Daily administration of artesunate (200 mg) for 14 days. | |
| NCT04098744/Recruiting | Cervical Neoplasia | A Phase II Double Blind, Placebo-controlled, Randomized Trial of Artesunate Vaginal Inserts for the Treatment of Patients With Cervical Intraepithelial Neoplasia (CIN2/3) | Participants will receive three 5-day cycles of artesunate inserts, 200 mg per day, at weeks 0, 2, and 4. | |
| Rutin | NCT00003365/Terminated | Colon Cancer | The Effect of Plant Phenolic Compounds on Human Colon Epithelial Cells | The administration of rutin was twice a day, for 6–10 weeks. Other phytocompounds (e.g. curcumin, quercetin) were evaluated in this study as well. |
| Ginseng | NCT00631852/Completed | Breast Cancer | A Phase II Biomarker Trial of Gelatin Encapsulated Extract of American Ginseng Root (LEAG) in Breast Cancer | The administration of American Ginseng Root extract was organised as follows: four 250 mg tablets daily for 5–14 days prior to surgery. |
| NCT02603016/Completed | Lung Neoplasm Breast Carcinoma | Phase 1 Study of Clinical Nutrition That Research Safty and Efficacy in Lung Neoplasms And Breast Carcinoma | Two tablets of Ginseng were administered by mouth, twice a day for 42 days. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehelean, C.A.; Marcovici, I.; Soica, C.; Mioc, M.; Coricovac, D.; Iurciuc, S.; Cretu, O.M.; Pinzaru, I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules 2021, 26, 1109. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26041109
Dehelean CA, Marcovici I, Soica C, Mioc M, Coricovac D, Iurciuc S, Cretu OM, Pinzaru I. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules. 2021; 26(4):1109. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26041109
Chicago/Turabian StyleDehelean, Cristina Adriana, Iasmina Marcovici, Codruta Soica, Marius Mioc, Dorina Coricovac, Stela Iurciuc, Octavian Marius Cretu, and Iulia Pinzaru. 2021. "Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy" Molecules 26, no. 4: 1109. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26041109