Extraction, Isolation and Characterization of Bioactive Compounds from Artemisia and Their Biological Significance: A Review
Abstract
:1. Introduction
Traditional and Current Uses of Artemisia Species
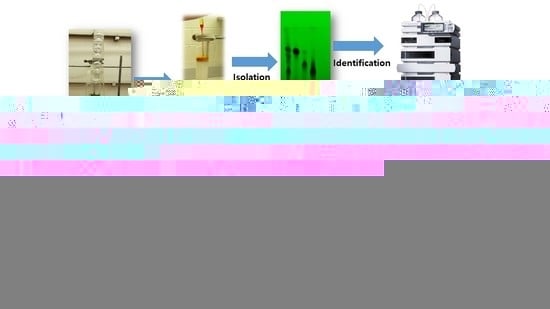
2. Extraction, Isolation, Characterization
2.1. Extraction Techniques
| Species Name | Origin | Method | Plant Part | Solvent System | Bioactive Constituent | Percent Yield (%) | Reference |
|---|---|---|---|---|---|---|---|
| Artemisia annua | Astore, Northern Areas Pakistan | Sonication | Flowers | 5 mL HPLC grade toluene | Artemisinin | 0.42 ± 0.03% | [35] |
| Artemisia annua | Astore, Northern Areas Pakistan | Sonication | Leaves | 5 mL HPLC grade toluene | Artemisinin | 0.44 ± 0.03% | [35] |
| Artemisia annua | Astore, Northern Area Pakistan | Sonication | Stems | 5 mL HPLC grade toluene | Artemisinin | 0.8 ± 0% | [35] |
| Artemisia dracunculus var dracunculus | Abbass Pur, Azad Kashmir Pakistan | Sonication | Leaves | 5 mL HPLC grade toluene | Artemisinin | 0.27 ± 0% | [35] |
| Artemisia dracunculus var dracunculus | Abbass Pur, Azad Kashmir Pakistan | Sonication | Stems | 5 mL HPLC grade toluene | Artemisinin | 0.12 ± 0.01% | [35] |
| Artemisia parviflora | Rawalakot, Azad Kashmir Pakistan | Sonication | Stems | 5 mL HPLC grade toluene | Artemisinin | 0.8 ± 0% | [35] |
| Artemisia moorcroftiana | Kalam, Swat Pakistan | Sonication | Stems | 5 mL HPLC grade toluene | Artemisinin | 0.8 ± 0% | [35] |
| Artemisia sieversiana | Soost, Northern Areas Pakistan | Sonication | Roots | 5 mL HPLC grade toluene | Artemisinin | 0.04 ± 0% | [35] |
| Artemisia sieversiana | Soost, Northern Areas Pakistan | Sonication | Stems | 5 mL HPLC grade toluene | Artemisinin | 0.8 ± 0% | [35] |
| Artemisia moorcroftiana | Kalam, Swat Pakistan | Sonication | Stems | 5 mL HPLC grade toluene | Artemisinin | 0.8 ± 0% | [35] |
| Artemisia vestita | Galyat, Pakistan | Sonication | Roots | 5 mL HPLC grade toluene | Artemisinin | 0.04 ± 0% | [35] |
| Artemisia vulgaris | Kalam, Swat Pakistan | Sonication | Flowers/leaves | 5 mL HPLC grade toluene | Artemisinin | 0.05–0.15% | [35] |
| Artemisia vulgaris | _ | silica gel column chromatography using gradient elution | Leaves | Ethyl acetate and dichloromethane | Yomogin and 1,2,3,4-diepoxy-11(13) eudesmen-12,8-olide | _ | [36] |
| Artemisia douglassiana | _ | Liquid-liquid extraction | Aerial parts | Ethyl acetate-hexane [1:9] | Dehydroleucodine | _ | [36] |
| Artemisia douglassiana | _ | Liquid-liquid extraction | Aerial parts | Silica gel with hexane-ethyl acetate mixtures | Dehydroparishin-B | _ | [36] |
| Artemisia diffusa | _ | Maceration | _ | n-hexane/ethyl acetate/methanol [1:1:1] | Tehranolide | _ | [36] |
| Artemisia princeps | _ | Liquid-liquid extraction | Aerial parts | Dichloromethane fraction column chromatography over silica gel using gradient elution of methanol and dichloromethane | Yomogin | _ | [36] |
| Artemisia ludoviciana | _ | Column chromatography | Aerial parts | Organic phase chromatographed repeatedly on normal-phase silica gel with ethyl acetate and hexane | Guaianolide ludartin | _ | [36] |
| Artemisia caerulescens ssp. cretacea | _ | Concentrated extract is extracted with boiling water. Aqueous solutions are extracted with chloroform | Flowers | Aluminum oxide column with 5% methanol in chloroform | Santonin | _ | [36] |
2.2. Isolation Techniques: TL
2.3. Characterization: LCMS & NMR
3. Bioactive Compounds from Artemisia
3.1. Phenolics & Flavonoids
3.2. Terpenoids
3.3. Coumarins
4. Biological Properties & Significance
4.1. Antitumor Activity
4.2. Anti-Inflammatory & Immunomodulatory Activity
4.3. Antiulcer Activity
4.4. Antimicrobial Activity
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brusotti, G.; Cesari, I.; Dentamaro, A.; Caccialanza, G.; Massolini, G. Isolation and Characterization of Bioactive Compounds from Plant Resources: The Role of Analysis in the Ethnopharmacological Approach. J. Pharm. Biomed. Anal. 2014, 87, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Bora, K.S.; Sharma, A. The Genus Artemisia: A Comprehensive Review. Pharm. Biol. 2011, 49, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Abad, M.J.; Bedoya, L.M.; Apaza, L.; Bermejo, P. The Artemisia L. Genus: A Review of Bioactive Essential Oils. Molecules 2012, 17, 2542–2566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weston, L.; Barney, J.; DiTommaso, A. A Review of the Biology and Ecology of Three Invasive Perennials in New York State: Japanese Knotweed (Polygonum cuspidatum), Mugwort (Artemisia vulgaris) and Pale Swallowwort (Vincetoxicum rossicum). Plant Soil 2005, 277, 53–69. [Google Scholar] [CrossRef]
- Abiri, R.; Silva, A.L.M.; de Mesquita, L.S.S.; de Mesquita, J.W.C.; Atabaki, N.; de Almeida, E.B.; Shaharuddin, N.A.; Malik, S. Towards a Better Understanding of Artemisia Vulgaris: Botany, Phytochemistry, Pharmacological and Biotechnological Potential. Food Res. Int. 2018, 109, 403–415. [Google Scholar] [CrossRef]
- Available online: https://plants.sc.egov.usda.gov/home (accessed on 11 November 2020).
- Datasheet Report for Artemisia biennis (Biennial Wormwood). Available online: https://www.cabi.org/isc/datasheetreport/112441 (accessed on 19 June 2020).
- Mojarrab, M.; Mehrabi, M.; Ahmadi, F.; Hosseinzadeh, L. Protective Effects of Fractions from Artemisia Biennis Hydro- Ethanolic Extract against Doxorubicin-Induced Oxidative Stress and Apoptosis in PC12 Cells. Iran. J. Basic Med. Sci. 2016, 19, 8. [Google Scholar]
- Biennial Wormwood (Artemisia biennis)|Idaho Fish and Game. Available online: https://idfg.idaho.gov/species/taxa/45280 (accessed on 22 December 2020).
- Ivanescu, B.; Lungu, C.; Vlase, L.; Gheldiu, A.M.; Grigorescu, C.; Corciova, A. Bioactive Compounds from Artemisia campestris L. Subsp. Campestris. Rev. Chim. 2018, 69, 3076–3081. [Google Scholar] [CrossRef]
- Dib, I.; El Alaoui-Faris, F.E. Artemisia Campestris L.: Review on Taxonomical Aspects, Cytogeography, Biological Activities and Bioactive Compounds. Biomed. Pharmacother. 2019, 109, 1884–1906. [Google Scholar] [CrossRef]
- Setzer, W.N.; Vogler, B.; Schmidt, J.M.; Leahy, J.G.; Rives, R. Antimicrobial Activity of Artemisia Douglasiana Leaf Essential Oil. Fitoterapia 2004, 75, 192–200. [Google Scholar] [CrossRef]
- Watson, L. CalPhotos: Artemisia douglasiana California Mugwort. Available online: https://calphotos.berkeley.edu/cgi/img_query? (accessed on 23 December 2020).
- Obolskiy, D.; Pischel, I.; Feistel, B.; Glotov, N.; Heinrich, M. Artemisia dracunculus L. (Tarragon): A Critical Review of Its Traditional Use, Chemical Composition, Pharmacology, and Safety. J. Agric. Food Chem. 2011, 59, 11367–11384. [Google Scholar] [CrossRef] [Green Version]
- Aglarova, A.M.; Zilfikarov, I.N.; Severtseva, O.V. Biological Characteristics and Useful Properties of Tarragon (Artemisia dracunculus L.) (Review). Pharm. Chem. J. 2008, 42, 81–86. [Google Scholar] [CrossRef]
- Artemisia dracunculus—Plant Finder. Available online: https://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=277563&isprofile=0& (accessed on 23 December 2020).
- Kelley, B.D.; Appelt, J.M.; Appelt, G.D. Artemisia Tridentata (Basin Sagebrush) in the Southwestern United States of America: Medicinal Uses and Pharmacologic Implications. Int. J. Addict. 1992, 27, 347–366. [Google Scholar] [CrossRef] [PubMed]
- Monroe, G.A. CalPhotos: Artemisia tridentata ssp. tridentata Basin Big Sagebrush. Available online: https://calphotos.berkeley.edu/cgi/img_query?enlarge=0000+0000+1102+0075 (accessed on 23 December 2020).
- Anupama. Mugwort (Artemisia vulgaris) Benefits, Uses, Cation and More. Available online: https://www.bimbima.com/health/artemisia-vulgaris/3726/ (accessed on 23 December 2020).
- Lee, S.-J.; Chung, H.-Y.; Maier, C.G.-A.; Wood, A.R.; Dixon, R.A.; Mabry, T.J. Estrogenic Flavonoids from Artemisia vulgaris L. J. Agric. Food Chem. 1998, 46, 3325–3329. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, F.; Zhou, X.-M.; Nui, C.-Y.; Lei, C.L. Repellent and Fumigant Activity of Essential Oil from Artemisia vulgaris to Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Available online: https://www.researchgate.net/publication/222325400_Repellent_and_fumigant_activity_of_essential_oil_from_Artemisia_vulgaris_toTribolium_castaneum_Herbst_Coleoptera_Tenebrionidae (accessed on 15 June 2020).
- Lee, J.K. Anti-Inflammatory Effects of Eriodictyol in Lipopolysaccharide stimulated Raw 264.7 Murine Macrophages. Arch. Pharm. Res. 2011, 34, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lazaro, M. Distribution and Biological Activities of the Flavonoid Luteolin. Mini-Rev. Med. Chem. 2009, 9, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.; Pajor, J.; Klin, P.; Rzepiela, A.; Ślesak, H.; Szopa, A. Significance of Artemisia vulgaris L.(Common Mugwort) in the history of medicine and its possible contemporary applications substantiated by phytochemical and pharmacological studies. Molecules 2020, 25, 4415. [Google Scholar] [CrossRef]
- Govindaraj, S.; Ranjitha Kumari, B.D. Composition and Larvicidal Activity of Artemisia vulgaris L. Stem Essential Oil against Aedes Aegypti. Jordan J. Biol. Sci. 2013, 6, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Judzentiene, A.; Garjonyte, R. Compositional Variability and Toxic Activity of Mugwort (Artemisia vulgaris) Essential Oils. Nat. Prod. Commun. 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Chandra, A.; Misra, L.N.; Thakur, R.S. Dihydrofurano-Terpenoids of Davana Oil. Tetrahedron Lett. 1987, 28, 6377–6380. [Google Scholar] [CrossRef]
- Haider, F.; Dwivedi, P.D.; Naqvi, A.A.; Bagchi, G.D. Essential Oil Composition of Artemisia Vulgaris Harvested at Different Growth Periods Under Indo-Gangetic Plain Conditions. J. Essent. Oil Res. 2003, 15, 376–378. [Google Scholar] [CrossRef]
- Judžentien, A. Chemical Composition of Essential Oils of Artemisia vulgaris L. (Mugwort) from North Lithuania. Chemija 2005, 17, 12–15. [Google Scholar]
- Sharmila, K.; Aadma, P. Effect of Artemisia vulgaris leaf extract on antioxidant status of primary chick embryo fibroblasts. IJPBS 2014, 5, 731–738. [Google Scholar]
- Haniya, A.K.; Padma, P. Phytochemical investigation of methanolic extract of Artemisia vulgaris L. leaves. IJPBS 2014, 5, 184–195. [Google Scholar]
- Afsar, S.; Kumar, K.R.; Gopal, J.V.; Raveesha, P. Assessment of antiinflammatory activity of Artemisia vulgaris leaves by cotton pellet granuloma method in Wistar albino rats. J. Pharm. Res. 2013, 7, 463–467. [Google Scholar]
- Gallego, R.; Montero, L.; Cifuentes, A.; Ibáñez, E.; Herrero, M. Green Extraction of Bioactive Compounds from Microalgae. J. Anal. Test. 2018, 2, 109–123. [Google Scholar] [CrossRef]
- Cao, J.; Yang, M.; Cao, F.; Wang, J.; Su, E. Well-Designed Hydrophobic Deep Eutectic Solvents as Green and Efficient Media for the Extraction of Artemisinin from Artemisia Annua Leaves. ACS Sustain. Chem. Eng. 2017, 5, 3270–3278. [Google Scholar] [CrossRef]
- Mannan, A.; Ahmed, I.; Arshad, W.; Asim, M.F.; Qureshi, R.A.; Hussain, I.; Mirza, B. Survey of Artemisinin Production by Diverse Artemisia Species in Northern Pakistan. Malar. J. 2010, 9, 310. [Google Scholar] [CrossRef] [Green Version]
- Ivanescu, B.; Miron, A.; Corciova, A. Sesquiterpene Lactones from Artemisia Genus: Biological Activities and Methods of Analysis. J. Anal. Methods Chem. 2015, 2015, 247685. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.; Latha, L. Extraction, Isolation and Characterization Of Bioactive Compounds From Plants’ Extracts. Afr. J. Tradit. Complement. Altern. Med. 2010, 8. [Google Scholar] [CrossRef] [Green Version]
- Zanousi, M.B.P.; Soleimani, T.; Keyhanfar, M.; Shirali, S.; Raeesi, M. Thin Layer Chromatography (TLC) Technique in the Investigation of Artemisinin Production in Artemisia annua L. Medicinal Plant Hairy Roots. J. Med. Plants Res. 2012, 6, 1842–1845. [Google Scholar]
- Sakipova, Z.; Wong, N.S.H.; Bekezhanova, T.; Sadykova Shukirbekova, A.; Boylan, F. Quantification of Santonin in Eight Species of Artemisia from Kazakhstan by Means of HPLC-UV: Method Development and Validation. PLoS ONE 2017, 12, e0173714. [Google Scholar]
- Tian, F.; Ruan, Q.-J.; Zhang, Y.; Cao, H.; Ma, Z.-G.; Zhou, G.-L.; Wu, M.-H. Quantitative Analysis of Six Phenolic Acids in Artemisia capillaris (Yinchen) by HPLC-DAD and Their Transformation Pathways in Decoction Preparation Process. Available online: https://www.hindawi.com/journals/jamc/2020/8950324/ (accessed on 13 July 2020).
- Wang, M.; Park, C.; Wu, Q.; Simon, J.E. Analysis of Artemisinin in Artemisia annua L. by LC-MS with Selected Ion Monitoring. J. Agric. Food Chem. 2005, 53, 7010–7013. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Bajpai, V.; Khandelwal, N.; Varshney, S.; Gaikwad, A.N.; Srivastava, M.; Singh, B.; Kumar, B. Determination of Bioactive Compounds of Artemisia Spp. Plant Extracts by LC–MS/MS Technique and Their in-Vitro Anti-Adipogenic Activity Screening. J. Pharm. Biomed. Anal. 2020, 193, 113707. [Google Scholar] [CrossRef]
- Nageeb, A.; Al-Tawashi, A.; Mohammad Emwas, A.-H.; Abdel-Halim Al-Talla, Z.; Al-Rifai, N. Comparison of Artemisia Annua Bioactivities between Traditional Medicine and Chemical Extracts. Curr. Bioact. Compd. 2013, 9, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.F.S.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as Antioxidants and Their Potential Synergism with Artemisinin against Malaria and Cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Serwah Boateng, N.A.; Ma, H. Latest Developments in Polyphenol Recovery and Purification from Plant By-Products: A Review. Trends Food Sci. Technol. 2020, 99, 375–388. [Google Scholar] [CrossRef]
- Springob, K.; Kutchan, T.M. Introduction to the Different Classes of Natural Products. In Plant-Derived Natural Products; Osbourn, A.E., Lanzotti, V., Eds.; Springer: New York, NY, USA, 2009; pp. 3–50. [Google Scholar]
- Batiha, G.E.S.; Olatunde, A.; El-Mleeh, A.; Hetta, H.F.; Al-Rejaie, S.; Alghamdi, S.; Zahoor, M.; Magdy Beshbishy, A.; Murata, T.; Zaragoza-Bastida, A.; et al. Bioactive compounds, pharmacological actions, and pharmacokinetics of wormwood (Artemisia absinthium). Antibiotics 2020, 9, 353. [Google Scholar] [CrossRef]
- Kim, M.H.; Seo, J.Y.; Liu, K.H.; Kim, J.S. Protective effect of Artemisia annua L. extract against galactose-induced oxidative stress in mice. PLoS ONE 2014, 9, e101486. [Google Scholar] [CrossRef] [Green Version]
- Barnabei, L.; Laplantine, E.; Mbongo, W.; Rieux-Laucat, F.; Weil, R. NF-κB: At the Borders of Autoimmunity and Inflammation. Front. Immunol. 2021, 12, 3169. [Google Scholar] [CrossRef]
- Foglio, M.A.; Dias, P.C.; Antônio, M.A.; Possenti, A.; Rodrigues, R.A.F.; da Silva, É.F.; Rehder, V.L.G.; de Carvalho, J.E. Antiulcerogenic Activity of Some Sesquiterpene Lactones Isolated from Artemisia Annua. Planta Med. 2002, 68, 515–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.Z.; Miller, L.H. The discovery of artemisinin and the Nobel Prize in Physiology or Medicine. Sci. China Life Sci. 2015, 58, 1175–1179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Hagens, C.; Walter-Sack, I.; Goeckenjan, M.; Osburg, J.; Storch-Hagenlocher, B.; Sertel, S. Prospective open uncontrolled phase I study to define a welltolerated dose of oral artesunate as add-on therapy in patients with metastatic breast cancer (ARTIC M33/2). Breast Cancer Res. Treat. 2017, 164, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, P.; Zhou, Y.; Chen, Z.; He, Y.; Mo, M. Dihydroartemisinin attenuates renal fibrosis through regulation of fibroblast proliferation and differentiation. Life Sci. 2019, 223, 29–37. [Google Scholar] [CrossRef]
- Sharma, B.N.; Marschall, M.; Henriksen, S.; Rinaldo, C.H. Antiviral effects of artesunate on polyomavirus BK replication in primary human kidney cells. Antimicrob. Agents Chemother. 2014, 58, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Xia, M.; Liu, D.; Liu, Y.; Liu, H. The therapeutic effect of artemisinin and its derivatives in kidney disease. Front. Pharmacol. 2020, 11, 380. [Google Scholar] [CrossRef]
- O’neill, P.M.; Barton, V.E.; Ward, S.A. The molecular mechanism of action of artemisinin—The debate continues. Molecules 2010, 15, 1705–1721. [Google Scholar] [CrossRef]
- Dong, Y.; Wittlin, S.; Sriraghavan, K.; Chollet, J.; Charman, S.A.; Charman, W.N.; Scheurer, C.; Urwyler, H.; Santo Tomas, J.; Snyder, C.; et al. The structure−activity relationship of the antimalarial ozonide Arterolane (OZ277). J. Med. Chem. 2010, 53, 481–491. [Google Scholar] [CrossRef]










| Artemisia Species Extract | Salient Applications | Animal Model and Cell Lines Used | Reference |
|---|---|---|---|
| Artemisia absinthium | Demonstrated reduction in the overall cholesterol level in diabetic rats through inhibition of enzyme activities involved in cholesterol biosynthesis | Alloxan-induced diabetic rats | [17,48] |
| Artemisia annua | Mice fed A. annua extract diet demonstrated diminished levels of malondialdehyde, a biomarker of lipid peroxidation. Studies demonstrated the use of A. annua extract has high anticancer effects | C57BL/6J mice Human Breast Adenocarcinoma MCF7 (BA), Human Lung Carcinoma (LC) and Chinese Hamster Ovary (CHO) cell lines and Primary Human Dermal Fibroblasts isolated from adult skin (HDFa) cells were used | [43,49] |
| Artemisia biennis | Fractions of A. biennis have been shown to stifle generation of reactive oxygen species (ROS) while improving the activity of superoxide dismutase (SOD). | PC12 Cells | [8] |
| Artemisia campestris | Antioxidant activity of A. campestris was revealed in a study that exposed rats to induced oxidative stress from a puffer fish. The rats were then fed aqueous extracts of A. campestris, resulting in the inhibition of thiobarbituric acid reactive substance (TBARS) and the amplification of antioxidant enzyme activities such as glutathione peroxidase (GSH-Px) and superoxide dismutase (SOD) in the brain, liver, and kidney. | Rats | [11] |
| Artemisia douglasiana | A. douglasiana leaf essential oil has been shown to be an efficacious complementary treatment for recurring urinary tract infection. | Studied in rodents | [14] |
| Artemisia dracunculus | A. dracunculus is typically used to enhance a poorly functioning digestive system by improving appetite to remove toxins from the body. In cultures with a particularly high intake of red meat, A. dracunculus is employed as a digestive stimulant. | Phenylbutazone-induced ulcer in rats | [14] |
| Artemisia tridentata | A. tridentata possesses anthelmintic activity which is useful in the treatment of oxyuriasis and ascariasis by inducing paralysis on the worm to flush out the parasite from the bowls. | - | [17] |
| Artemisia vulgaris | The antihypertensive activity of A. vulgaris has been demonstrated to limit the hypertensive effects of noradrenaline Cytotoxic activity inhibits growth of tumor cells in cancer cell lines | Isolated perfused rat mesentery SW-480, MCF7, HL-60, HeLa, 293T, and A7R5. | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anibogwu, R.; Jesus, K.D.; Pradhan, S.; Pashikanti, S.; Mateen, S.; Sharma, K. Extraction, Isolation and Characterization of Bioactive Compounds from Artemisia and Their Biological Significance: A Review. Molecules 2021, 26, 6995. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26226995
Anibogwu R, Jesus KD, Pradhan S, Pashikanti S, Mateen S, Sharma K. Extraction, Isolation and Characterization of Bioactive Compounds from Artemisia and Their Biological Significance: A Review. Molecules. 2021; 26(22):6995. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26226995
Chicago/Turabian StyleAnibogwu, Rosemary, Karl De Jesus, Samjhana Pradhan, Srinath Pashikanti, Sameena Mateen, and Kavita Sharma. 2021. "Extraction, Isolation and Characterization of Bioactive Compounds from Artemisia and Their Biological Significance: A Review" Molecules 26, no. 22: 6995. https://0-doi-org.brum.beds.ac.uk/10.3390/molecules26226995