Recent Advances in Transition Metal Dichalcogenide Cathode Materials for Aqueous Rechargeable Multivalent Metal-Ion Batteries
Abstract
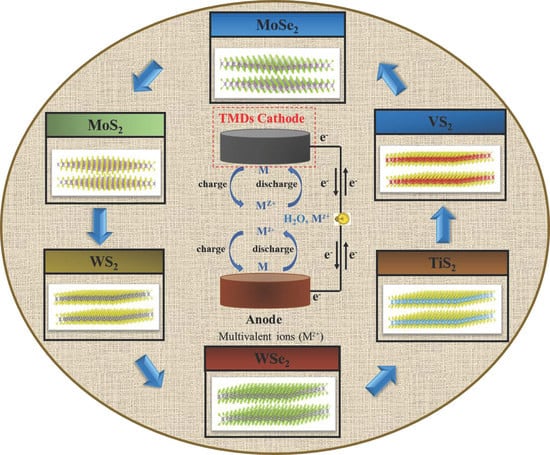
:1. Introduction
| Charge Carrier Ions | Zn2+ | Mg2+ | Al3+ | Ca2+ | Li+ | Na+ | K+ |
|---|---|---|---|---|---|---|---|
| Atomic mass (g mol−1) | 65.41 | 24.31 | 26.98 | 40.08 | 6.94 | 22.99 | 39.1 |
| Density (at 20 °C) (g cm−3) | 7.11 | 1.74 | 2.7 | 1.55 | 0.53 | 0.97 | 0.89 |
| Crystal structure | Hexagonal | Hexagonal | face-centered cubic | face-centered cubic | body-centered cubic | body-centered cubic | body-centered cubic |
| Abundance a | 25 | 8 | 3 | 5 | 33 | 6 | 7 |
| Metal cost (USD kg−1) | 2.2 | 2.2 | 1.9 | 2.28 | 19.2 | 3.1 | 13.1 |
| Ionic radius (Å) | 0.75 | 0.72 | 0.53 | 1.00 | 0.76 | 1.02 | 1.38 |
| Hydrated ionic radius (Å) | 4.3 | 4.28 | 4.75 | 4.12 | 3.82 | 3.58 | 3.31 |
| Redox potential vs. SHE | −0.763 | −2.356 | −1.676 | −2.84 | −3.04 | −2.713 | −2.924 |
| Gravimetric specific capacity of metal anode (mAh g−1) | 820 | 2206 | 2980 | 1337 | 3860 | 1166 | 685 |
| Volumetric specific capacity (mAh cm−1) | 5855 | 3834 | 8046 | 2072 | 2061 | 1129 | 610 |
2. Brief Introduction of Transition Metal Dichalcogenides (TMDs)
2.1. Concept and Principle of TMDs
2.2. Advantages and Challenges of TMDs
3. TMD Cathode Materials for Multivalent Metal-Ion Batteries (MMIBs)
3.1. Zinc-Ion Batteries (ZIBs)

3.2. Magnesium-Ion Batteries (MIBs)

3.3. Aluminum-Ion Batteries (AIBs)
4. Modification Strategies for TMDs toward High-Performance Aqueous Multivalent Metal-Ion Batteries (AMMIBs)
4.1. Interlayer Modification
4.1.1. Intercalation of Hydrophilic Species into TMDs
4.1.2. Exfoliation
4.2. Defect Modification
4.3. Hybridization with Carbon
4.4. Phase Modification
5. Conclusion and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Jiao, Y.; Kang, L.; Berry-Gair, J.; McColl, K.; Li, J.; Dong, H.; Jiang, H.; Wang, R.; Corà, F.; Brett, D.J.L.; et al. Enabling stable MnO2 matrix for aqueous zinc-ion battery cathodes. J. Mater. Chem. A 2020, 8, 22075–22082. [Google Scholar] [CrossRef]
- Pan, H.; Shao, Y.; Yan, P.; Cheng, Y.; Han, K.S.; Nie, Z.; Wang, C.; Yang, J.; Li, X.; Bhattacharya, P.; et al. Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat. Energy 2016, 1, 16039. [Google Scholar] [CrossRef]
- Zampardi, G.; La Mantia, F. Prussian blue analogues as aqueous Zn-ion batteries electrodes: Current challenges and future perspectives. Curr. Opin. Electrochem. 2020, 21, 84–92. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Winter, M.; Barnett, B.; Xu, K. Before Li Ion Batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef] [PubMed]
- Mun, Y.S.; Pham, T.N.; Hoang Bui, V.K.; Tanaji, S.T.; Lee, H.U.; Lee, G.-W.; Choi, J.S.; Kim, I.T.; Lee, Y.-C. Tin oxide evolution by heat-treatment with tin-aminoclay (SnAC) under argon condition for lithium-ion battery (LIB) anode applications. J. Power Sources 2019, 437, 226946. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Park, D.; Kim, I.T. Fe(x)Sn(y)O(z) Composites as Anode Materials for Lithium-Ion Storage. J. Nanosci. Nanotechnol. 2019, 19, 6636–6640. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.; Kim, J.H.; Kim, I.T. Electrochemical Performance of Sn/SnO/Ni3Sn Composite Anodes for Lithium-Ion Batteries. J. Nanosci. Nanotechnol. 2019, 19, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.; Tanaji, S.T.; Choi, J.-S.; Lee, H.U.; Kim, I.T.; Lee, Y.-C. Preparation of Sn-aminoclay (SnAC)-templated Fe3O4 nanoparticles as an anode material for lithium-ion batteries. RSC Adv. 2019, 9, 10536–10545. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.P.; Kim, I.T. Ag Nanoparticle-Decorated MoS(2) Nanosheets for Enhancing Electrochemical Performance in Lithium Storage. Nanomaterials 2021, 11, 626. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, I.T. Self-Assembled Few-Layered MoS(2) on SnO(2) Anode for Enhancing Lithium-Ion Storage. Nanomaterials 2020, 10, 2558. [Google Scholar] [CrossRef]
- Preman, A.N.; Lee, H.; Yoo, J.; Kim, I.T.; Saito, T.; Ahn, S.-k. Progress of 3D network binders in silicon anodes for lithium ion batteries. J. Mater. Chem. A 2020, 8, 25548–25570. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, I.T. W2C/WS2 Alloy Nanoflowers as Anode Materials for Lithium-Ion Storage. Nanomaterials 2020, 10, 1336. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Vo, T.N.; Kim, I.T. GeTe-TiC-C Composite Anodes for Li-Ion Storage. Materials 2020, 13, 4222. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.N.; Kim, D.S.; Mun, Y.S.; Lee, H.J.; Ahn, S.-K.; Kim, I.T. Fast charging sodium-ion batteries based on Te-P-C composites and insights to low-frequency limits of four common equivalent impedance circuits. Chem. Eng. J. 2020, 398, 125703. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef]
- Pramudita, J.C.; Sehrawat, D.; Goonetilleke, D.; Sharma, N. An Initial Review of the Status of Electrode Materials for Potassium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602911. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, J.; Xu, Z.J.; Zhang, S.; Zhang, Q. Recent progress in metal–organic polymers as promising electrodes for lithium/sodium rechargeable batteries. J. Mater. Chem. A 2019, 7, 4259–4290. [Google Scholar] [CrossRef]
- Wang, S.; Sun, C.; Wang, N.; Zhang, Q. Ni- and/or Mn-based layered transition metal oxides as cathode materials for sodium ion batteries: Status, challenges and countermeasures. J. Mater. Chem. A 2019, 7, 10138–10158. [Google Scholar] [CrossRef]
- Xiong, W.; Huang, W.; Zhang, M.; Hu, P.; Cui, H.; Zhang, Q. Pillar[5]quinone–Carbon Nanocomposites as High-Capacity Cathodes for Sodium-Ion Batteries. Chem. Mater. 2019, 31, 8069–8075. [Google Scholar] [CrossRef]
- Ponrouch, A.; Bitenc, J.; Dominko, R.; Lindahl, N.; Johansson, P.; Palacin, M.R. Multivalent rechargeable batteries. Energy Storage Mater. 2019, 20, 253–262. [Google Scholar] [CrossRef]
- Song, M.; Tan, H.; Chao, D.; Fan, H.J. Recent Advances in Zn-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1802564. [Google Scholar] [CrossRef]
- Liu, W.; Hao, J.; Xu, C.; Mou, J.; Dong, L.; Jiang, F.; Kang, Z.; Wu, J.; Jiang, B.; Kang, F. Investigation of zinc ion storage of transition metal oxides, sulfides, and borides in zinc ion battery systems. Chem. Commun. 2017, 53, 6872–6874. [Google Scholar] [CrossRef]
- Wang, H.; Yu, D.; Kuang, C.; Cheng, L.; Li, W.; Feng, X.; Zhang, Z.; Zhang, X.; Zhang, Y. Alkali Metal Anodes for Rechargeable Batteries. Chem 2019, 5, 313–338. [Google Scholar] [CrossRef] [Green Version]
- Stoddart, A. Alkali metal batteries: Preventing failure. Nat. Rev. Mater. 2018, 3, 18021. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Han, C.; Wang, Z.; Liu, Z.; Tang, Z.; Zhi, C. Advanced rechargeable zinc-based batteries: Recent progress and future perspectives. Nano Energy 2019, 62, 550–587. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Pan, A.; Liang, S. Recent Advances in Aqueous Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2480–2501. [Google Scholar] [CrossRef]
- Konarov, A.; Voronina, N.; Jo, J.H.; Bakenov, Z.; Sun, Y.-K.; Myung, S.-T. Present and Future Perspective on Electrode Materials for Rechargeable Zinc-Ion Batteries. ACS Energy Lett. 2018, 3, 2620–2640. [Google Scholar] [CrossRef]
- Selvakumaran, D.; Pan, A.; Liang, S.; Cao, G. A review on recent developments and challenges of cathode materials for rechargeable aqueous Zn-ion batteries. J. Mater. Chem. A 2019, 7, 18209–18236. [Google Scholar] [CrossRef]
- Muldoon, J.; Bucur, C.B.; Gregory, T. Quest for Nonaqueous Multivalent Secondary Batteries: Magnesium and Beyond. Chem. Rev. 2014, 114, 11683–11720. [Google Scholar] [CrossRef]
- Cai, T.; Zhao, L.; Hu, H.; Li, T.; Li, X.; Guo, S.; Li, Y.; Xue, Q.; Xing, W.; Yan, Z.; et al. Stable CoSe2/carbon nanodice@reduced graphene oxide composites for high-performance rechargeable aluminum-ion batteries. Energy Environ. Sci. 2018, 11, 2341–2347. [Google Scholar] [CrossRef]
- Gummow, R.J.; Vamvounis, G.; Kannan, M.B.; He, Y. Calcium-Ion Batteries: Current State-of-the-Art and Future Perspectives. Adv. Mater. 2018, 30, 1801702. [Google Scholar] [CrossRef]
- Yu, X.; Prévot, M.S.; Guijarro, N.; Sivula, K. Self-assembled 2D WSe2 thin films for photoelectrochemical hydrogen production. Nat. Commun. 2015, 6, 7596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Wang, L.; Li, Y.; Zhang, F.; Lin, L.; Niu, S.; Chenet, D.; Zhang, X.; Hao, Y.; Heinz, T.F.; et al. Piezoelectricity of single-atomic-layer MoS2 for energy conversion and piezotronics. Nature 2014, 514, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chao, D.; Liu, E.; Jaroniec, M.; Zhao, N.; Qiao, S.-Z. Transition metal dichalcogenides for alkali metal ion batteries: Engineering strategies at the atomic level. Energy Environ. Sci. 2020, 13, 1096–1131. [Google Scholar] [CrossRef]
- Yuan, H.; Kong, L.; Li, T.; Zhang, Q. A review of transition metal chalcogenide/graphene nanocomposites for energy storage and conversion. Chin. Chem. Lett. 2017, 28, 2180–2194. [Google Scholar] [CrossRef]
- Voiry, D.; Yang, J.; Chhowalla, M. Recent Strategies for Improving the Catalytic Activity of 2D TMD Nanosheets toward the Hydrogen Evolution Reaction. Adv. Mater. 2016, 28, 6197–6206. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Choi, W.; Choudhary, N.; Han, G.H.; Park, J.; Akinwande, D.; Lee, Y.H. Recent development of two-dimensional transition metal dichalcogenides and their applications. Mater. Today 2017, 20, 116–130. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, J.; Zhang, W.; Lee, C.-S. Interlayer Nanoarchitectonics of Two-Dimensional Transition-Metal Dichalcogenides Nanosheets for Energy Storage and Conversion Applications. Adv. Energy Mater. 2017, 7, 1700571. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Zeng, Z.; Zhang, H. Metal dichalcogenide nanosheets: Preparation, properties and applications. Chem. Soc. Rev. 2013, 42, 1934–1946. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Hao, J.; Wang, Z.; Mao, J.; Guo, Z. Recent progress and perspectives on aqueous Zn-based rechargeable batteries with mild aqueous electrolytes. Energy Storage Mater. 2019, 20, 410–437. [Google Scholar] [CrossRef]
- Huang, S.; Du, P.; Min, C.; Liao, Y.; Sun, H.; Jiang, Y. Poly(1-amino-5-chloroanthraquinone): Highly Selective and Ultrasensitive Fluorescent Chemosensor For Ferric Ion. J. Fluoresc. 2013, 23, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Cheng, X.; Yu, H.; Zhu, H.; Peng, N.; Zheng, R.; Zhang, J.; Shui, M.; Cui, Y.; Shu, J. An overview and future perspectives of aqueous rechargeable polyvalent ion batteries. Energy Storage Mater. 2019, 18, 68–91. [Google Scholar] [CrossRef]
- Li, Q.; Bjerrum, N.J. Aluminum as anode for energy storage and conversion: A review. J. Power Sources 2002, 110, 1–10. [Google Scholar] [CrossRef]
- Wu, S.; Du, Y.; Sun, S. Transition metal dichalcogenide based nanomaterials for rechargeable batteries. Chem. Eng. J. 2017, 307, 189–207. [Google Scholar] [CrossRef]
- Yun, Q.; Li, L.; Hu, Z.; Lu, Q.; Chen, B.; Zhang, H. Layered Transition Metal Dichalcogenide-Based Nanomaterials for Electrochemical Energy Storage. Adv. Mater. 2020, 32, 1903826. [Google Scholar] [CrossRef]
- Rashad, M.; Asif, M.; Wang, Y.; He, Z.; Ahmed, I. Recent advances in electrolytes and cathode materials for magnesium and hybrid-ion batteries. Energy Storage Mater. 2020, 25, 342–375. [Google Scholar] [CrossRef]
- Jellinek, F. Transition metal chalcogenides. relationship between chemical composition, crystal structure and physical properties. React. Solids 1988, 5, 323–339. [Google Scholar] [CrossRef]
- Stephenson, T.; Li, Z.; Olsen, B.; Mitlin, D. Lithium ion battery applications of molybdenum disulfide (MoS2) nanocomposites. Energy Environ. Sci. 2014, 7, 209–231. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, H.; Chang, W.-H.; Shin, H.; Li, L. Synthesis and structure of two-dimensional transition-metal dichalcogenides. MRS Bull. 2015, 40, 566–576. [Google Scholar] [CrossRef]
- Voiry, D.; Mohite, A.; Chhowalla, M. Phase engineering of transition metal dichalcogenides. Chem. Soc. Rev. 2015, 44, 2702–2712. [Google Scholar] [CrossRef]
- Han, S.A.; Bhatia, R.; Kim, S.-W. Synthesis, properties and potential applications of two-dimensional transition metal dichalcogenides. Nano Converg. 2015, 2, 17. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Liu, H.; Qu, J.; Li, J. Two-dimensional layered MoS2: Rational design, properties and electrochemical applications. Energy Environ. Sci. 2016, 9, 1190–1209. [Google Scholar] [CrossRef]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2, 17033. [Google Scholar] [CrossRef]
- Wei, Z.; Li, B.; Xia, C.; Cui, Y.; He, J.; Xia, J.-B.; Li, J. Various Structures of 2D Transition-Metal Dichalcogenides and Their Applications. Small Methods 2018, 2, 1800094. [Google Scholar] [CrossRef]
- Zhang, Q.; Mei, L.; Cao, X.; Tang, Y.; Zeng, Z. Intercalation and exfoliation chemistries of transition metal dichalcogenides. J. Mater. Chem. A 2020, 8, 15417–15444. [Google Scholar] [CrossRef]
- Lee, W.S.V.; Xiong, T.; Wang, X.; Xue, J. Unraveling MoS2 and Transition Metal Dichalcogenides as Functional Zinc-Ion Battery Cathode: A Perspective. Small Methods 2021, 5, 2000815. [Google Scholar] [CrossRef]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and optoelectronics of two-dimensional transition metal dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Coogan, Á.; Gun’ko, Y.K. Solution-based “bottom-up” synthesis of group VI transition metal dichalcogenides and their applications. Mater. Adv. 2021, 2, 146–164. [Google Scholar] [CrossRef]
- Song, I.; Park, C.; Choi, H.C. Synthesis and properties of molybdenum disulphide: From bulk to atomic layers. RSC Adv. 2015, 5, 7495–7514. [Google Scholar] [CrossRef] [Green Version]
- Eda, G.; Yamaguchi, H.; Voiry, D.; Fujita, T.; Chen, M.; Chhowalla, M. Photoluminescence from Chemically Exfoliated MoS2. Nano Lett. 2011, 11, 5111–5116. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Giustiniano, F.; Eda, G. Electronic transport properties of transition metal dichalcogenide field-effect devices: Surface and interface effects. Chem. Soc. Rev. 2015, 44, 7715–7736. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-Based Materials: Synthesis, Characterization, Properties, and Applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Zhang, H. Ultrathin Two-Dimensional Nanomaterials. ACS Nano 2015, 9, 9451–9469. [Google Scholar] [CrossRef]
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015, 44, 2713–2731. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D Transition-Metal-Dichalcogenide-Nanosheet-Based Composites for Photocatalytic and Electrocatalytic Hydrogen Evolution Reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lei, W.; Zhang, S.; Liu, Y.; Wallace, G.G.; Chen, J. Two-dimensional transition metal dichalcogenides in supercapacitors and secondary batteries. Energy Storage Mater. 2019, 19, 408–423. [Google Scholar] [CrossRef]
- Yu, X.; Yun, S.; Yeon, J.S.; Bhattacharya, P.; Wang, L.; Lee, S.W.; Hu, X.; Park, H.S. Emergent Pseudocapacitance of 2D Nanomaterials. Adv. Energy Mater. 2018, 8, 1702930. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; Zhang, W.; Qiao, Y.; Cao, X.; Xie, X.; Zhou, H.; Pan, A.; Liang, S. Tuning Interface Bridging between MoSe2 and Three-Dimensional Carbon Framework by Incorporation of MoC Intermediate to Boost Lithium Storage Capability. Nano-Micro Lett. 2020, 12, 171. [Google Scholar] [CrossRef]
- Tansel, B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: Hydrated radius, hydration free energy and viscous effects. Sep. Purif. Technol. 2012, 86, 119–126. [Google Scholar] [CrossRef]
- Liu, J.; Xu, P.; Liang, J.; Liu, H.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Boosting aqueous zinc-ion storage in MoS2 via controllable phase. Chem. Eng. J. 2020, 389, 124405. [Google Scholar] [CrossRef]
- Ming, J.; Guo, J.; Xia, C.; Wang, W.; Alshareef, H.N. Zinc-ion batteries: Materials, mechanisms, and applications. Mater. Sci. Eng. R Rep. 2019, 135, 58–84. [Google Scholar] [CrossRef]
- Liang, H.; Cao, Z.; Ming, F.; Zhang, W.; Anjum, D.H.; Cui, Y.; Cavallo, L.; Alshareef, H.N. Aqueous Zinc-Ion Storage in MoS2 by Tuning the Intercalation Energy. Nano Lett. 2019, 19, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Q.; Mo, F.; Liang, G.; Liu, Z.; Tang, Z.; Ma, L.; Liu, J.; Shi, Z.; Zhi, C. MoS2 nanosheets with expanded interlayer spacing for rechargeable aqueous Zn-ion batteries. Energy Storage Mater. 2019, 19, 94–101. [Google Scholar] [CrossRef]
- Xu, W.; Sun, C.; Zhao, K.; Cheng, X.; Rawal, S.; Xu, Y.; Wang, Y. Defect engineering activating (Boosting) zinc storage capacity of MoS2. Energy Storage Mater. 2019, 16, 527–534. [Google Scholar] [CrossRef]
- Yang, Y. Synthesis and Properties of Halloysite Templated Tubular MoS2 as Cathode Material for Rechargeable Aqueous Zn-ion Batteries. Int. J. Electrochem. Sci. 2020, 15, 6052–6059. [Google Scholar] [CrossRef]
- He, P.; Yan, M.; Zhang, G.; Sun, R.; Chen, L.; An, Q.; Mai, L. Layered VS2 Nanosheet-Based Aqueous Zn Ion Battery Cathode. Adv. Energy Mater. 2017, 7, 1601920. [Google Scholar] [CrossRef]
- Jiao, T.; Yang, Q.; Wu, S.; Wang, Z.; Chen, D.; Shen, D.; Liu, B.; Cheng, J.; Li, H.; Ma, L.; et al. Binder-free hierarchical VS2 electrodes for high-performance aqueous Zn ion batteries towards commercial level mass loading. J. Mater. Chem. A 2019, 7, 16330–16338. [Google Scholar] [CrossRef]
- Wu, Z.; Lu, C.; Wang, Y.; Zhang, L.; Jiang, L.; Tian, W.; Cai, C.; Gu, Q.; Sun, Z.; Hu, L. Ultrathin VSe2 Nanosheets with Fast Ion Diffusion and Robust Structural Stability for Rechargeable Zinc-Ion Battery Cathode. Small 2020, 16, 2000698. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Xiao, Q.; Zhang, B.; Yan, Z.; Liu, X.; Chen, S.; Ren, Z.; Yu, Y. VS4 with a chain crystal structure used as an intercalation cathode for aqueous Zn-ion batteries. J. Mater. Chem. A 2020, 8, 10761–10766. [Google Scholar] [CrossRef]
- Aurbach, D.; Lu, Z.; Schechter, A.; Gofer, Y.; Gizbar, H.; Turgeman, R.; Cohen, Y.; Moshkovich, M.; Levi, E. Prototype systems for rechargeable magnesium batteries. Nature 2000, 407, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, G.; Jiang, H.; Parkin, I.P.; Shearing, P.R.; Brett, D.J.L. Cathode Design for Aqueous Rechargeable Multivalent Ion Batteries: Challenges and Opportunities. Adv. Funct. Mater. 2021, 31, 2010445. [Google Scholar] [CrossRef]
- Yang, S.; Li, D.; Zhang, T.; Tao, Z.; Chen, J. First-Principles Study of Zigzag MoS2 Nanoribbon as a Promising Cathode Material for Rechargeable Mg Batteries. J. Phys. Chem. C 2012, 116, 1307–1312. [Google Scholar] [CrossRef]
- Liang, Y.; Feng, R.; Yang, S.; Ma, H.; Liang, J.; Chen, J. Rechargeable Mg Batteries with Graphene-like MoS2 Cathode and Ultrasmall Mg Nanoparticle Anode. Adv. Mater. 2011, 23, 640–643. [Google Scholar] [CrossRef]
- Liu, Y.; Jiao, L.; Wu, Q.; Du, J.; Zhao, Y.; Si, Y.; Wang, Y.; Yuan, H. Sandwich-structured graphene-like MoS2/C microspheres for rechargeable Mg batteries. J. Mater. Chem. A 2013, 1, 5822–5826. [Google Scholar] [CrossRef]
- Truong, Q.D.; Kempaiah Devaraju, M.; Nakayasu, Y.; Tamura, N.; Sasaki, Y.; Tomai, T.; Honma, I. Exfoliated MoS2 and MoSe2 Nanosheets by a Supercritical Fluid Process for a Hybrid Mg–Li-Ion Battery. ACS Omega 2017, 2, 2360–2367. [Google Scholar] [CrossRef] [Green Version]
- Mao, M.; Ji, X.; Hou, S.; Gao, T.; Wang, F.; Chen, L.; Fan, X.; Chen, J.; Ma, J.; Wang, C. Tuning Anionic Chemistry to Improve Kinetics of Mg Intercalation. Chem. Mater. 2019, 31, 3183–3191. [Google Scholar] [CrossRef]
- Gu, Y.; Katsura, Y.; Yoshino, T.; Takagi, H.; Taniguchi, K. Rechargeable magnesium-ion battery based on a TiSe2-cathode with d-p orbital hybridized electronic structure. Sci. Rep. 2015, 5, 12486. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Wei, Z.; Zhang, S.; Wang, X.; Wang, Y.; He, M.; Huang, K. Hierarchical WSe2 nanoflower as a cathode material for rechargeable Mg-ion batteries. J. Colloid Interface Sci. 2021, 588, 378–383. [Google Scholar] [CrossRef]
- Guduru, R.K.; Icaza, J.C. A Brief Review on Multivalent Intercalation Batteries with Aqueous Electrolytes. Nanomaterials 2016, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, J.J.; Yan, N.F.; Pan, G.L.; Li, G.R.; Gao, X.P. Aluminum storage behavior of anatase TiO2 nanotube arrays in aqueous solution for aluminum ion batteries. Energy Environ. Sci. 2012, 5, 9743–9746. [Google Scholar] [CrossRef]
- He, S.; Wang, J.; Zhang, X.; Chen, J.; Wang, Z.; Yang, T.; Liu, Z.; Liang, Y.; Wang, B.; Liu, S.; et al. A High-Energy Aqueous Aluminum-Manganese Battery. Adv. Funct. Mater. 2019, 29, 1905228. [Google Scholar] [CrossRef]
- Lahan, H.; Boruah, R.; Hazarika, A.; Das, S.K. Anatase TiO2 as an Anode Material for Rechargeable Aqueous Aluminum-Ion Batteries: Remarkable Graphene Induced Aluminum Ion Storage Phenomenon. J. Phys. Chem. C 2017, 121, 26241–26249. [Google Scholar] [CrossRef]
- He, Y.J.; Peng, J.F.; Chu, W.; Li, Y.Z.; Tong, D.G. Retracted Article: Black mesoporous anatase TiO2 nanoleaves: A high capacity and high rate anode for aqueous Al-ion batteries. J. Mater. Chem. A 2014, 2, 1721–1731. [Google Scholar] [CrossRef]
- Liu, S.; Pan, G.L.; Li, G.R.; Gao, X.P. Copper hexacyanoferrate nanoparticles as cathode material for aqueous Al-ion batteries. J. Mater. Chem. A 2015, 3, 959–962. [Google Scholar] [CrossRef]
- Zhou, A.; Jiang, L.; Yue, J.; Tong, Y.; Zhang, Q.; Lin, Z.; Liu, B.; Wu, C.; Suo, L.; Hu, Y.-S.; et al. Water-in-Salt Electrolyte Promotes High-Capacity FeFe(CN)6 Cathode for Aqueous Al-Ion Battery. ACS Appl. Mater. Interfaces 2019, 11, 41356–41362. [Google Scholar] [CrossRef]
- Zhao, Q.; Zachman, M.J.; Al Sadat, W.I.; Zheng, J.; Kourkoutis, L.F.; Archer, L. Solid electrolyte interphases for high-energy aqueous aluminum electrochemical cells. Sci. Adv. 2018, 4, eaau8131. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Gu, S.; Zhang, Q.; Bai, Y.; Li, M.; Yuan, Y.; Wang, H.; Liu, X.; Yuan, Y.; Zhu, N.; et al. Electrochemically activated spinel manganese oxide for rechargeable aqueous aluminum battery. Nat. Commun. 2019, 10, 73. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Y.; Zhang, Y.; Kwok, H.Y.H.; Wu, M.; Zhao, X.; Leung, D.Y.C. A low-cost and dendrite-free rechargeable aluminium-ion battery with superior performance. J. Mater. Chem. A 2019, 7, 17420–17425. [Google Scholar] [CrossRef]
- Fan, X.; Gaddam, R.R.; Kumar, N.A.; Zhao, X.S. A Hybrid Mg2+/Li+ Battery Based on Interlayer-Expanded MoS2/Graphene Cathode. Adv. Energy Mater. 2017, 7, 1700317. [Google Scholar] [CrossRef]
- Li, Z.; Niu, B.; Liu, J.; Li, J.; Kang, F. Rechargeable Aluminum-Ion Battery Based on MoS2 Microsphere Cathode. ACS Appl. Mater. Interfaces 2018, 10, 9451–9459. [Google Scholar] [CrossRef]
- Divya, S.; Johnston, J.H.; Nann, T. Molybdenum Dichalcogenide Cathodes for Aluminum-Ion Batteries. Energy Technol. 2020, 8, 2000038. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Sun, R.; Xiong, F.; Pei, C.; Han, K.; Peng, C.; Fan, Y.; Yang, W.; An, Q.; Mai, L. A rechargeable aluminum-ion battery based on a VS2 nanosheet cathode. Phys. Chem. Chem. Phys. 2018, 20, 22563–22568. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.; Scheifers, J.P.; Fu, C.; Zhang, J.; Fokwa, B.P.T.; Guo, J. Titanium Sulfides as Intercalation-Type Cathode Materials for Rechargeable Aluminum Batteries. ACS Appl. Mater. Interfaces 2017, 9, 21251–21257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, L.; Lv, G.; Xing, X.; Guo, J. Reversible Electrochemical Intercalation of Aluminum in Mo6S8. Chem. Mater. 2015, 27, 4926–4929. [Google Scholar] [CrossRef]
- Kundu, D.; Adams, B.D.; Duffort, V.; Vajargah, S.H.; Nazar, L.F. A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode. Nat. Energy 2016, 1, 16119. [Google Scholar] [CrossRef]
- Bin, D.; Huo, W.; Yuan, Y.; Huang, J.; Liu, Y.; Zhang, Y.; Dong, F.; Wang, Y.; Xia, Y. Organic-Inorganic-Induced Polymer Intercalation into Layered Composites for Aqueous Zinc-Ion Battery. Chem 2020, 6, 968–984. [Google Scholar] [CrossRef]
- Jung, Y.; Zhou, Y.; Cha, J.J. Intercalation in two-dimensional transition metal chalcogenides. Inorg. Chem. Front. 2016, 3, 452–463. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, A.; Wu, X.; van de Groep, J.; Tang, P.; Li, S.; Liu, B.; Shi, F.; Wan, J.; Li, Q.; et al. Reversible and selective ion intercalation through the top surface of few-layer MoS2. Nat. Commun. 2018, 9, 5289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.; Zhu, Q.; Shi, Y.; Chen, H.; Zhang, D.; Du, Z.; Yang, S. Single Zinc Atoms Immobilized on MXene (Ti3C2Clx) Layers toward Dendrite-Free Lithium Metal Anodes. ACS Nano 2020, 14, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.-Y.; Sun, X. Interlayer Engineering of Layered Cathode Materials for Advanced Zn Storage. Chem 2020, 6, 817–819. [Google Scholar] [CrossRef]
- Winchester, A.; Ghosh, S.; Feng, S.; Elias, A.L.; Mallouk, T.; Terrones, M.; Talapatra, S. Electrochemical Characterization of Liquid Phase Exfoliated Two-Dimensional Layers of Molybdenum Disulfide. ACS Appl. Mater. Interfaces 2014, 6, 2125–2130. [Google Scholar] [CrossRef]
- Bang, G.S.; Nam, K.W.; Kim, J.Y.; Shin, J.; Choi, J.W.; Choi, S.-Y. Effective Liquid-Phase Exfoliation and Sodium Ion Battery Application of MoS2 Nanosheets. ACS Appl. Mater. Interfaces 2014, 6, 7084–7089. [Google Scholar] [CrossRef]
- Coleman, J.N.; Lotya, M.; O’Neill, A.; Bergin, S.D.; King, P.J.; Khan, U.; Young, K.; Gaucher, A.; De, S.; Smith, R.J.; et al. Two-Dimensional Nanosheets Produced by Liquid Exfoliation of Layered Materials. Science 2011, 331, 568. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.J.; King, P.J.; Lotya, M.; Wirtz, C.; Khan, U.; De, S.; O’Neill, A.; Duesberg, G.S.; Grunlan, J.C.; Moriarty, G.; et al. Large-Scale Exfoliation of Inorganic Layered Compounds in Aqueous Surfactant Solutions. Adv. Mater. 2011, 23, 3944–3948. [Google Scholar] [CrossRef] [PubMed]
- Shmeliov, A.; Shannon, M.; Wang, P.; Kim, J.S.; Okunishi, E.; Nellist, P.D.; Dolui, K.; Sanvito, S.; Nicolosi, V. Unusual Stacking Variations in Liquid-Phase Exfoliated Transition Metal Dichalcogenides. ACS Nano 2014, 8, 3690–3699. [Google Scholar] [CrossRef]
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Rangappa, D.; Sone, K.; Wang, M.; Gautam, U.K.; Golberg, D.; Itoh, H.; Ichihara, M.; Honma, I. Rapid and Direct Conversion of Graphite Crystals into High-Yielding, Good-Quality Graphene by Supercritical Fluid Exfoliation. Chem. A Eur. J. 2010, 16, 6488–6494. [Google Scholar] [CrossRef] [PubMed]
- Rangappa, D.; Murukanahally, K.D.; Tomai, T.; Unemoto, A.; Honma, I. Ultrathin Nanosheets of Li2MSiO4 (M = Fe, Mn) as High-Capacity Li-Ion Battery Electrode. Nano Lett. 2012, 12, 1146–1151. [Google Scholar] [CrossRef]
- Truong, Q.D.; Devaraju, M.K.; Honma, I. Benzylamine-directed growth of olivine-type LiMPO4 nanoplates by a supercritical ethanol process for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 17400–17407. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Tang, C.; Wang, J.; Zhang, Q.; Wang, Y.; Zhang, J. Atomic Modulation and Structure Design of Carbons for Bifunctional Electrocatalysis in Metal–Air Batteries. Adv. Mater. 2019, 31, 1803800. [Google Scholar] [CrossRef] [PubMed]
- Uchaker, E.; Cao, G. The Role of Intentionally Introduced Defects on Electrode Materials for Alkali-Ion Batteries. Chem. Asian J. 2015, 10, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, L.; Tao, L.; Lu, Y.; Wang, S. Defect-Based Single-Atom Electrocatalysts. Small Methods 2019, 3, 1800406. [Google Scholar] [CrossRef]
- Banhart, F.; Kotakoski, J.; Krasheninnikov, A.V. Structural Defects in Graphene. ACS Nano 2011, 5, 26–41. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.; Bueken, B.; De Vos, D.E.; Fischer, R.A. Defect-Engineered Metal–Organic Frameworks. Angew. Chem. Int. Ed. 2015, 54, 7234–7254. [Google Scholar] [CrossRef] [Green Version]
- Lin, Z.; Carvalho, B.R.; Kahn, E.; Lv, R.; Rao, R.; Terrones, H.; Pimenta, M.A.; Terrones, M. Defect engineering of two-dimensional transition metal dichalcogenides. 2D Mater. 2016, 3, 022002. [Google Scholar] [CrossRef]
- Matsunaga, K.; Tanaka, T.; Yamamoto, T.; Ikuhara, Y. First-principles calculations of intrinsic defects in Al2O3. Phys. Rev. B 2003, 68, 085110. [Google Scholar] [CrossRef]
- Meggiolaro, D.; Mosconi, E.; De Angelis, F. Formation of Surface Defects Dominates Ion Migration in Lead-Halide Perovskites. ACS Energy Lett. 2019, 4, 779–785. [Google Scholar] [CrossRef] [Green Version]
- Paier, J.; Penschke, C.; Sauer, J. Oxygen Defects and Surface Chemistry of Ceria: Quantum Chemical Studies Compared to Experiment. Chem. Rev. 2013, 113, 3949–3985. [Google Scholar] [CrossRef]
- Wilby, A.; Neale, D. Defects Introduced into Metals during Fabrication and Service. 2011. Available online: http://www.eolss.net/Sample-Chapters/C05/E6-36-04-01.pdf (accessed on 1 March 2012).
- Xiong, T.; Yu, Z.G.; Wu, H.; Du, Y.; Xie, Q.; Chen, J.; Zhang, Y.-W.; Pennycook, S.J.; Lee, W.S.V.; Xue, J. Defect Engineering of Oxygen-Deficient Manganese Oxide to Achieve High-Performing Aqueous Zinc Ion Battery. Adv. Energy Mater. 2019, 9, 1803815. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Luo, Z.; Huang, X.; Tan, C.; Li, H.; Zheng, B.; Li, B.; Huang, Y.; Yang, J.; et al. Liquid-phase growth of platinum nanoparticles on molybdenum trioxide nanosheets: An enhanced catalyst with intrinsic peroxidase-like catalytic activity. Nanoscale 2014, 6, 12340–12344. [Google Scholar] [CrossRef]
- Deng, Z.; Jiang, H.; Li, C. 2D Metal Chalcogenides Incorporated into Carbon and their Assembly for Energy Storage Applications. Small 2018, 14, 1800148. [Google Scholar] [CrossRef]
- Lou, X.W.; Li, C.M.; Archer, L.A. Designed Synthesis of Coaxial SnO2@carbon Hollow Nanospheres for Highly Reversible Lithium Storage. Adv. Mater. 2009, 21, 2536–2539. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Liu, N.; Lian, Y. Carbonaceous microspheres prepared by hydrothermal carbonization of glucose for direct use in catalytic dehydration of fructose. RSC Adv. 2015, 5, 17526–17531. [Google Scholar] [CrossRef]
- Chen, Y.; Song, B.; Tang, X.; Lu, L.; Xue, J. Ultrasmall Fe3O4 Nanoparticle/MoS2 Nanosheet Composites with Superior Performances for Lithium Ion Batteries. Small 2014, 10, 1536–1543. [Google Scholar] [CrossRef]
- Gong, Y.; Yang, S.; Zhan, L.; Ma, L.; Vajtai, R.; Ajayan, P.M. A Bottom-Up Approach to Build 3D Architectures from Nanosheets for Superior Lithium Storage. Adv. Funct. Mater. 2014, 24, 125–130. [Google Scholar] [CrossRef]
- Zhu, C.; Mu, X.; van Aken, P.A.; Yu, Y.; Maier, J. Single-Layered Ultrasmall Nanoplates of MoS2 Embedded in Carbon Nanofibers with Excellent Electrochemical Performance for Lithium and Sodium Storage. Angew. Chem. Int. Ed. 2014, 53, 2152–2156. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Zhang, J.; Li, H.; Tang, Z.; Wang, X. Nanosheet-assembled MoSe2 and S-doped MoSe2−x nanostructures for superior lithium storage properties and hydrogen evolution reactions. Inorg. Chem. Front. 2015, 2, 931–937. [Google Scholar] [CrossRef]
- Fan, X.; Xu, P.; Li, Y.C.; Zhou, D.; Sun, Y.; Nguyen, M.A.T.; Terrones, M.; Mallouk, T.E. Controlled Exfoliation of MoS2 Crystals into Trilayer Nanosheets. J. Am. Chem. Soc. 2016, 138, 5143–5149. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Jiang, D.-E. Mechanism of Hydrogen Evolution Reaction on 1T-MoS2 from First Principles. ACS Catal. 2016, 6, 4953–4961. [Google Scholar] [CrossRef]
- Lei, Z.; Zhan, J.; Tang, L.; Zhang, Y.; Wang, Y. Recent Development of Metallic (1T) Phase of Molybdenum Disulfide for Energy Conversion and Storage. Adv. Energy Mater. 2018, 8, 1703482. [Google Scholar] [CrossRef]
- Wang, R.; Yu, Y.; Zhou, S.; Li, H.; Wong, H.; Luo, Z.; Gan, L.; Zhai, T. Strategies on Phase Control in Transition Metal Dichalcogenides. Adv. Funct. Mater. 2018, 28, 1802473. [Google Scholar] [CrossRef]
- Yin, Y.; Han, J.; Zhang, Y.; Zhang, X.; Xu, P.; Yuan, Q.; Samad, L.; Wang, X.; Wang, Y.; Zhang, Z.; et al. Contributions of Phase, Sulfur Vacancies, and Edges to the Hydrogen Evolution Reaction Catalytic Activity of Porous Molybdenum Disulfide Nanosheets. J. Am. Chem. Soc. 2016, 138, 7965–7972. [Google Scholar] [CrossRef]
- Tan, C.; Luo, Z.; Chaturvedi, A.; Cai, Y.; Du, Y.; Gong, Y.; Huang, Y.; Lai, Z.; Zhang, X.; Zheng, L.; et al. Preparation of High-Percentage 1T-Phase Transition Metal Dichalcogenide Nanodots for Electrochemical Hydrogen Evolution. Adv. Mater. 2018, 30, 1705509. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, T.; Juan, P.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Hierarchical Cobalt Borate/MXenes Hybrid with Extraordinary Electrocatalytic Performance in Oxygen Evolution Reaction. ChemSusChem 2018, 11, 3758–3765. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Xu, D.; Zhu, Y.; Peng, W.; Li, Y.; Zhang, F.; Fan, X. Hierarchical “nanoroll” like MoS2/Ti3C2Tx hybrid with high electrocatalytic hydrogen evolution activity. Appl. Catal. B Environ. 2019, 241, 89–94. [Google Scholar] [CrossRef]
- Chang, K.; Hai, X.; Pang, H.; Zhang, H.; Shi, L.; Liu, G.; Liu, H.; Zhao, G.; Li, M.; Ye, J. Targeted Synthesis of 2H- and 1T-Phase MoS2 Monolayers for Catalytic Hydrogen Evolution. Adv. Mater. 2016, 28, 10033–10041. [Google Scholar] [CrossRef] [PubMed]












| TMDs | Interlayer Spacing of Activated (Å) | Specific Capacity (mAh g−1) | Capacity Retention (%) | Cycle | Current Density (mA g−1) | Voltage Ranges (V) | Comments (Main Findings) | Ref. |
|---|---|---|---|---|---|---|---|---|
| MoS2 | - | 18.0 | - | 50 | 50 | 0.1–1.9 | Study of zinc ion storage in pristine TMDs | [24] |
| WS2 | - | 22.0 | - | 50 | 50 | 0.1–1.9 | Study of zinc ion storage in pristine TMDs | [24] |
| MoS2-O | 9.5 | 232 | - | 20 | 100 | 0.2–1.4 | Reduction in intercalation energy barrier by oxygen incorporation | [77] |
| E-MoS2 | 7.0 | 202.6 | 98.6 | 600 | 100 | 0.3–1.5 | A novel structure of MoS2 | [78] |
| 1T-MoS2 | 6.8 | - | 98.4 | 400 | 100 | 0.25–1.25 | Effect of different phase contents on the distinct performance | [75] |
| Defect-rich MoS2 | 6.86 | 88.6 | 87.8 | 1000 | 100 | 0.25–1.25 | Development of defect rich MoS2 nanosheets | [79] |
| Tubular MoS2 | 6.5 | 146.2 | 74 | 800 | 200 | 0.25–1.25 | A novel structure of MoS2 | [80] |
| VS2 | 5.76 | 110.9 | 98 | 200 | 500 | 0.4–1.0 | Storage mechanism of Zn/VS2 | [81] |
| VS2@SS | 5.8 | 198 | 80 | 2000 | 2000 | 0.4–1.0 | Binder-free hierarchical VS2@SS electrode | [82] |
| VSe2 | 6.1 | 131.8 | 80.8 | 500 | 100 | 0.25–1.50 | Zinc-ion transport behavior in VSe2 nanosheets | [83] |
| VS4 | 5.83 | 110 | 85 | 500 | 2500 | 0.2–1.6 | Energy storage mechanism of VS4 | [84] |
| TMDs | Interlayer Spacing of Activated (Å) | Specific Capacity (mAh g−1) | Capacity Retention (%) | Cycle | Current Density (mA g−1) | Voltage Range (V) | Comments (Main Findings) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Zigzag MoS2 | - | 170 | - | - | - | - | Study of Mg adsorption sites with DFT calculations | [87] |
| G-WS2 | 0.7 | 161.5 | 95 | 50 | 20 | 0.5–3.0 | A novel structure of G-MoS2 cathode and ultra-small Mg nanoparticle anode | [88] |
| MoS2/C | 1.07 | 118.8 | - | 50 | 50 | 0–2.4 | A novel structure of MoS2/C cathode and AZ31 Mg alloy anode | [89] |
| Bulk MoS2 | - | 81 | 68.7 | 10 | 20 | 0.2–2.2 | Exfoliation of TMD into high-quality nanosheets | [90] |
| Bulk MoSe2 | - | 55 | 73.3 | 5 | 20 | 0.2–2.2 | Exfoliation of TMD into high-quality nanosheets | [90] |
| TiS2 | - | 80 | 63.0 | 40 | 5 | 0.5–2.0 | Kinetic study of Mg2+ migration in layered TMD | [91] |
| TiSe2 | - | 86 | 74.8 | 40 | 5 | 0.5–2.0 | Kinetic study of Mg2+ migration in layered TMD | [91] |
| 1T-TiSe2 | - | 108 | - | 40 | - | 0.25–1.8 | Study of phase effect in TiSe2 on the battery performance | [92] |
| WSe2 | 6.8 | 239 | 91.9 | 100 | 50 | 0–2.5 | A novel WSe2 structure | [93] |
| TMDs | Interlayer Spacing of Activated (Å) | Specific Capacity (mAh g−1) | Capacity Retention (%) | Cycle | Current Density (mA g−1) | Voltage Range (V) | Comments (Main Findings) | Ref. |
|---|---|---|---|---|---|---|---|---|
| TiS2 | - | 70 | - | 50 | 5 | 0.2–1.3 | Reversible insertion and extraction of Al in TiS2 | [108] |
| MoS2 (E-MG) | 1.0 | 87.6 | - | 120 | 20 | 0.01–2.0 | A novel structure of MoS2 (E-MG) | [104] |
| MoS2 | 6.2 | 66.7 | - | 100 | 40 | 0.5–2.0 | Phase transition mechanism during the charge-discharge process in MoS2 | [105] |
| MoS2 | 6.3 | 30 | - | - | 100 | 0.01–2.5 | Intercalation mechanism of Al3+ into MoS2 | [106] |
| G-VS2 | 5.75 | 50 | 33.6 | 50 | 100 | 0.3–1.7 | Intercalation mechanism of Al3+ into G-VS2 | [107] |
| Mo6S8 | - | 70 | - | 50 | - | 0.1–1.2 | Reversible intercalation and extraction of Al3+ in Mo6S8 | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang Huy, V.P.; Ahn, Y.N.; Hur, J. Recent Advances in Transition Metal Dichalcogenide Cathode Materials for Aqueous Rechargeable Multivalent Metal-Ion Batteries. Nanomaterials 2021, 11, 1517. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061517
Hoang Huy VP, Ahn YN, Hur J. Recent Advances in Transition Metal Dichalcogenide Cathode Materials for Aqueous Rechargeable Multivalent Metal-Ion Batteries. Nanomaterials. 2021; 11(6):1517. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061517
Chicago/Turabian StyleHoang Huy, Vo Pham, Yong Nam Ahn, and Jaehyun Hur. 2021. "Recent Advances in Transition Metal Dichalcogenide Cathode Materials for Aqueous Rechargeable Multivalent Metal-Ion Batteries" Nanomaterials 11, no. 6: 1517. https://0-doi-org.brum.beds.ac.uk/10.3390/nano11061517