Presence and Levels of Galactosyllactoses and Other Oligosaccharides in Human Milk and Their Variation during Lactation and According to Maternal Phenotype
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Milk Samples
2.2. Analysis of Human Milk Oligosaccharides
2.3. Selection of Human Milk Oligosaccharides
2.4. Maternal Secretor- and Lewis Phenotype (HM Group)
2.5. Data Presentation and Statistical Analysis
3. Results
3.1. Human Milk Samples
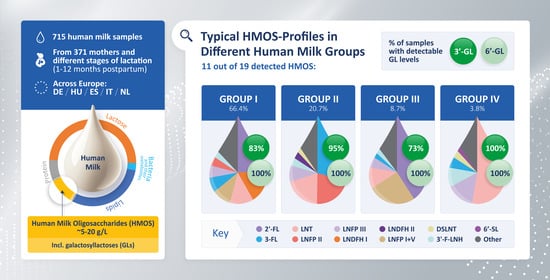
3.2. Maternal Secretor- and Lewis Phenotype (HM Group)
3.3. Presence of HMOS According to Maternal Secretor- and Lewis Phenotype and Lactation Stage
3.4. Relative Levels of HMOS According to Maternal Secretor- and Lewis Phenotype and Lactation Stage
3.5. Absolute Concentrations of GLs according to Maternal Secretor- and Lewis Phenotype and Lactation Stage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Thurl, S.; Munzert, M.; Boehm, G.; Matthews, C.; Stahl, B. Systematic review of the concentrations of oligosaccharides in human milk. Nutr. Rev. 2017, 75, 920–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunz, C.; Meyer, C.; Collado, M.C.; Geiger, L.; García-Mantrana, I.; Bertua-Ríos, B.; Martínez-Costa, C.; Borsch, C.; Rudloff, S. Influence of Gestational Age, Secretor, and Lewis Blood Group Status on the Oligosaccharide Content of Human Milk. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Elwakiel, M.; Hageman, J.A.; Wang, W.; Szeto, I.M.; van Goudoever, J.B.; Hettinga, K.A.; Schols, H.A. Human Milk Oligosaccharides in Colostrum and Mature Milk of Chinese Mothers: Lewis Positive Secretor Subgroups. J. Agric. Food Chem. 2018, 66, 7036–7043. [Google Scholar] [CrossRef]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Müller-Werner, B.; Jelinek, J.; Stahl, B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, A.L.; Alves, R.; Figueiredo, A.; Alves-Santos, N.; Freitas-Costa, N.; Batalha, M.; Yonemitsu, C.; Manivong, N.; Furst, A.; Bode, L.; et al. Human Milk Oligosaccharide Profile Variation Throughout Postpartum in Healthy Women in a Brazilian Cohort. Nutrients 2020, 12, 790. [Google Scholar] [CrossRef] [Green Version]
- McGuire, M.K.; Meehan, C.L.; McGuire, M.A.; Williams, J.E.; Foster, J.; Sellen, D.W.; Kamau-Mbuthia, E.W.; Kamundia, E.W.; Mbugua, S.; Moore, S.E.; et al. What’s normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically. Am. J. Clin. Nutr. 2017, 105, 1086–1100. [Google Scholar] [CrossRef]
- Ayechu-Muruzabal, V.; van Stigt, A.H.; Mank, M.; Willemsen, L.E.M.; Stahl, B.; Garssen, J.; Van’t Land, B. Diversity of Human Milk Oligosaccharides and Effects on Early Life Immune Development. Front. Pediatr. 2018, 6, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R.; et al. Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef]
- Gabrielli, O.; Zampini, L.; Galeazzi, T.; Padella, L.; Santoro, L.; Peila, C.; Giuliani, F.; Bertino, E.; Fabris, C.; Coppa, G.V. Preterm milk oligosaccharides during the first month of lactation. Pediatrics 2011, 128, e1520–e1531. [Google Scholar] [CrossRef] [PubMed]
- Tonon, K.M.; de Morais, M.B.; Abrao, A.F.V.; Miranda, A.; Morais, T.B. Maternal and Infant Factors Associated with Human Milk Oligosaccharides Concentrations According to Secretor and Lewis Phenotypes. Nutrients 2019, 11, 1358. [Google Scholar] [CrossRef] [Green Version]
- Thurl, S.; Henker, J.; Siegel, M.; Tovar, K.; Sawatzki, G. Detection of four human milk groups with respect to Lewis blood group dependent oligosaccharides. Glycoconj. J. 1997, 14, 795–799. [Google Scholar] [CrossRef]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [Green Version]
- Kunz, C.; Rudloff, S.; Baier, W.; Klein, N.; Strobel, S. Oligosaccharides in human milk: Structural, functional, and metabolic aspects. Annu. Rev. Nutr. 2000, 20, 699–722. [Google Scholar] [CrossRef] [PubMed]
- Oriol, R.; Le Pendu, J.; Mollicone, R. Genetics of ABO, H, Lewis, X and related antigens. Vox Sang. 1986, 51, 161–171. [Google Scholar] [CrossRef]
- Stahl, B.; Thurl, S.; Henker, J.; Siegel, M.; Finke, B.; Sawatzki, G. Detection of four human milk groups with respect to Lewis-blood-group-dependent oligosaccharides by serologic and chromatographic analysis. Adv. Exp. Med. Biol. 2001, 501, 299–306. [Google Scholar] [PubMed]
- Sprenger, N.; Lee, L.Y.; De Castro, C.A.; Steenhout, P.; Thakkar, S.K. Longitudinal change of selected human milk oligosaccharides and association to infants’ growth, an observatory, single center, longitudinal cohort study. PLoS ONE 2017, 12, e0171814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musilova, S.; Rada, V.; Vlkova, E.; Bunesova, V. Beneficial effects of human milk oligosaccharides on gut microbiota. Benef. Microbes 2014, 5, 273–283. [Google Scholar] [CrossRef]
- Bering, S.B. Human Milk Oligosaccharides to Prevent Gut Dysfunction and Necrotizing Enterocolitis in Preterm Neonates. Nutrients 2018, 10, 1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Grimm, R.; German, J.B.; Lebrilla, C.B. Annotation and structural analysis of sialylated human milk oligosaccharides. J. Proteome Res. 2011, 10, 856–868. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Tao, N.; German, J.B.; Grimm, R.; Lebrilla, C.B. Development of an annotated library of neutral human milk oligosaccharides. J. Proteome Res. 2010, 9, 4138–4151. [Google Scholar] [CrossRef] [Green Version]
- Stahl, B.; Thurl, S.; Zeng, J.; Karas, M.; Hillenkamp, F.; Steup, M.; Sawatzki, G. Oligosaccharides from human milk as revealed by matrix-assisted laser desorption/ionization mass spectrometry. Anal. Biochem. 1994, 223, 218–226. [Google Scholar] [CrossRef]
- Urashima, T.; Kitaoka, M.; Terabayashi, T.; Fukuda, K.; Ohnishi, M.; Kobata, A. Milk Oligosaccharides. In Oligosaccharides: Sources, Properties and Applications; Gordon, N.G., Ed.; Nova Science Publishers: New York, NY, USA, 2011; pp. 1–58. [Google Scholar]
- Urashima, T.; Hirabayashi, J.; Sato, S.; Kobata, A. Human Milk Oligosaccharides as Essential Tools for Basic and Application Studies on Galectins. Trends Glycosci. Glycotechnol. 2018, 30, SJ11–SJ24. [Google Scholar] [CrossRef] [Green Version]
- Ruhaak, L.R.; Lebrilla, C.B. Advances in analysis of human milk oligosaccharides. Adv. Nutr. 2012, 3, 406s–414s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newburg, D.S.; Ko, J.S.; Leone, S.; Nanthakumar, N.N. Human Milk Oligosaccharides and Synthetic Galactosyloligosaccharides Contain 3′-, 4-, and 6′-Galactosyllactose and Attenuate Inflammation in Human T84, NCM-460, and H4 Cells and Intestinal Tissue Ex Vivo. J. Nutr. 2016, 146, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Sumiyoshi, W.; Urashima, T.; Nakamura, T.; Arai, I.; Saito, T.; Tsumura, N.; Wang, B.; Brand-Miller, J.; Watanabe, Y.; Kimura, K. Determination of each neutral oligosaccharide in the milk of Japanese women during the course of lactation. Br. J. Nutr. 2003, 89, 61–69. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, S.; Leone, S.; Newburg, D.S. Human colostrum oligosaccharides modulate major immunologic pathways of immature human intestine. Mucosal Immunol. 2014, 7, 1326–1339. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lawlor, N.T.; Newburg, D.S. Human Milk Components Modulate Toll-Like Receptor-Mediated Inflammation. Adv. Nutr. 2016, 7, 102–111. [Google Scholar] [CrossRef] [Green Version]
- Mank, M.; Hauner, H.; Heck, A.J.R.; Stahl, B. Targeted LC-ESI-MS(2) characterization of human milk oligosaccharide diversity at 6 to 16 weeks post-partum reveals clear staging effects and distinctive milk groups. Anal. Bioanal. Chem. 2020, 412, 6887–6907. [Google Scholar] [CrossRef]
- Vriezinga, S.L.; Auricchio, R.; Bravi, E.; Castillejo, G.; Chmielewska, A.; Crespo Escobar, P.; Kolacek, S.; Koletzko, S.; Korponay-Szabo, I.R.; Mummert, E.; et al. Randomized feeding intervention in infants at high risk for celiac disease. N. Engl. J. Med. 2014, 371, 1304–1315. [Google Scholar] [CrossRef] [Green Version]
- Hogen Esch, C.E.; Rosen, A.; Auricchio, R.; Romanos, J.; Chmielewska, A.; Putter, H.; Ivarsson, A.; Szajewska, H.; Koning, F.; Wijmenga, C.; et al. The PreventCD Study design: Towards new strategies for the prevention of coeliac disease. Eur. J. Gastroenterol. Hepatol. 2010, 22, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Van de Heijning, B.J.M.; Stahl, B.; Schaart, M.W.; van der Beek, E.M.; Rings, E.H.H.M.; Mearin, M.L. Fatty acid and amino acid content and composition of human milk in the course of lactation. Adv. Ped. Res. 2017, 4, 16. [Google Scholar]
- Kottler, R.; Mank, M.; Hennig, R.; Müller-Werner, B.; Stahl, B.; Reichl, U.; Rapp, E. Development of a high-throughput glycoanalysis method for the characterization of oligosaccharides in human milk utilizing multiplexed capillary gel electrophoresis with laser-induced fluorescence detection. Electrophoresis 2013, 34, 2323–2336. [Google Scholar] [CrossRef]
- Ullsten, S.; Danielsson, R.; Backstrom, D.; Sjoberg, P.; Bergquist, J. Urine profiling using capillary electrophoresis-mass spectrometry and multivariate data analysis. J. Chromatogr. A. 2006, 1117, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Blank, D.; Gebhardt, S.; Maass, K.; Lochnit, G.; Dotz, V.; Blank, J.; Geyer, R.; Kunz, C. High-throughput mass finger printing and Lewis blood group assignment of human milk oligosaccharides. Anal. Bioanal. Chem. 2011, 401, 2495–2510. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Strategy for Infant and Young Child Feeding; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Ballard, O.; Morrow, A.L. Human Milk Composition. Nutrients and Bioactive Factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durham, S.D.; Robinson, R.C.; Olga, L.; Ong, K.K.; Chichlowski, M.; Dunger, D.B.; Barile, D. A one-year study of human milk oligosaccharide profiles in the milk of healthy UK mothers and their relationship to maternal FUT2 genotype. Glycobiology 2021, cwab057. [Google Scholar] [CrossRef]
- Samuel, T.M.; Binia, A.; de Castro, C.A.; Thakkar, S.K.; Billeaud, C.; Agosti, M.; Al-Jashi, I.; Costeira, M.J.; Marchini, G.; Martinez-Costa, C.; et al. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci. Rep. 2019, 9, 11767. [Google Scholar] [CrossRef]
- Siziba, L.P.; Mank, M.; Stahl, B.; Gonsalves, J.; Blijenberg, B.; Rothenbacher, D.; Genuneit, J. Human milk oligosaccharide profiles over 12 months of lactation: The Ulm SPATZ Health Study. Nutrients 2021, 13, 1973. [Google Scholar] [CrossRef]
- Zimmermann, D.W. Inflation of Type I Error Rates by Unequal Variances Associated with Parametric, Nonparametric, and Rank-Transformation Tests. Psicológica 2004, 25, 103–133. [Google Scholar]



 , results from postpartum months 8 to 12 are based on samples from <10 participants and should be interpreted with caution.
, results from postpartum months 8 to 12 are based on samples from <10 participants and should be interpreted with caution.
 , results from postpartum months 8 to 12 are based on samples from <10 participants and should be interpreted with caution.
, results from postpartum months 8 to 12 are based on samples from <10 participants and should be interpreted with caution.

 , results from postpartum months 8 to 12 are based on samples from <10 participants and should be interpreted with caution.
, results from postpartum months 8 to 12 are based on samples from <10 participants and should be interpreted with caution.
 , results from postpartum months 8 to 12 are based on samples from <10 participants and should be interpreted with caution.
, results from postpartum months 8 to 12 are based on samples from <10 participants and should be interpreted with caution.
| HM Group | Specific Fucosylation | Prominent α1-2, α1-3 and α1-4 Fucosylated HMOS | |
|---|---|---|---|
| α1-2 (Se) | α1-4 (Le) | ||
| I Lewis (a − b + c − d−) 1 | + | + | 2′-FL, 3-FL, DFL, LNFP I, LNFP II, LNFP III, LNDFH I and LNDFH II |
| II Lewis (a + b − c − d−) 1 | − | + | 3-FL, LNFP II, LNFP III and LNDFH II |
| III Lewis (a − b − c − d +) 1 | + | − | 2′-FL, 3-FL, DFL, LNFP I and LNFP III |
| IV Lewis (a − b − c + d−) 1 | − | − | 3-FL and LNFP III |
| HM Group I (n = 420 Samples, 241 Donors) Median (Q1–Q3) | HM Group II (n = 137 Samples, 83 Donors) Median (Q1–Q3) | HM Group III (n = 53 Samples, 32 Donors) Median (Q1–Q3) | HM Group IV (n = 22 Samples, 14 Donors) Median (Q1–Q3) | |
|---|---|---|---|---|
| nTPA 1 | 12.3 (10.0–17.6) a | 10.7 (8.33–14.1) a,b | 10.9 (8.73–14.7) a,b | 8.18 (5.75–10.5) b |
| rPA (%) | ||||
| 2′-FL | 23.2 (18.4–29.0) a | 0.00 (0.00–0.00) 2,b | 40.9 (34.2–48.9) c | 0.00 (0.00–0.00) 2,b |
| 3-FL | 7.33 (3.70–13.8) a | 28.4 (17.2–35.8) b | 0.00 (0.00–0.00) 2,c | 6.25 (4.03–11.3) a |
| DFL | 1.61 (1.13–2.20) a | 0.00 (0.00–0.00) 2,b | 0.65 (0.33–1.08) c | 0.00 (0.00–0.00) 2,b |
| LNT | 11.9 (9.18–14.6) a | 19.1 (14.5–26.5) b | 11.8 (9.10–13.8) a | 51.8 (46.4–56.7) c |
| LNnT | 1.44 (1.02–2.04) a | 0.68 (0.45–1.16) b | 1.10 (0.82–1.50) c | 0.91 (0.45–1.44) a,b,c |
| LNFP I + V | 9.69 (6.00–14.0) a | 1.65 (1.39–1.97) b | 24.9 (19.3–31.5) c | 1.60 (1.32–2.36) b |
| LNFP II | 5.13 (3.57–7.34) a | 20.6 (17.6–25.4) b | 0.00 (0.00–0.00) 2,c | 0.00 (0.00–0.00) 2,c |
| LNFP III | 6.81 (4.62–8.83) a | 9.25 (7.30–11.3) b | 6.44 (3.85–8.49) a | 14.3 (9.58–18.0) c |
| LNDFH I | 17.3 (14.9–20.2) a | 0.00 (0.00–0.00) 2,b | 0.00 (0.00–0.00) 2,b | 0.00 (0.00–0.00) 2,b |
| LNDFH II | 0.00 (0.00–0.00) 2,a | 3.77 (2.84–4.88) b | 0.00 (0.00–0.00)2,a | 0.00 (0.00–0.00) 2,a |
| 3′-SL | 0.89 (0.70–1.07) a | 1.01 (0.82–1.25) b | 0.94 (0.76–1.22) a,b | 1.45 (1.09–1.63) c |
| 6′-SL | 1.22 (0.76–2.22) a | 1.58 (0.92–2.67) a,b | 1.31 (0.88–2.51) a | 2.42 (1.80–3.45) b |
| DSLNT | 1.71 (1.32–2.27) a | 2.37 (1.69–3.13) b | 1.94 (1.31–2.48) a,b | 4.09 (3.22–5.05) c |
| LSTa | 0.17 (0.00–0.26) a | 0.21 (0.00–0.31) a | 0.00 (0.00–0.32) a | 0.47 (0.32–0.75) b |
| LSTb | 0.80 (0.60–1.04) a | 1.21 (1.03–1.49) b | 0.75 (0.57–0.89) a | 2.21 (1.89–2.75) c |
| LSTc | 0.75 (0.42–1.37) a | 0.75 (0.39–1.32) a | 0.60 (0.45–1.42) a | 1.01 (0.74–1.79) a |
| 3′-F-LNH | 3.42 (2.35–4.59) a | 4.60 (2.71–7.18) b | 2.65 (1.99–4.72) a | 10.5 (8.48–11.3) c |
| 2′-F-LNH | 0.00 (0.00–0.00) 2,a | 0.00 (0.00–0.00) 2,a | 0.00 (0.00–1.20) a | 0.00 (0.00–0.00) 2,a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eussen, S.R.B.M.; Mank, M.; Kottler, R.; Hoffmann, X.-K.; Behne, A.; Rapp, E.; Stahl, B.; Mearin, M.L.; Koletzko, B. Presence and Levels of Galactosyllactoses and Other Oligosaccharides in Human Milk and Their Variation during Lactation and According to Maternal Phenotype. Nutrients 2021, 13, 2324. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13072324
Eussen SRBM, Mank M, Kottler R, Hoffmann X-K, Behne A, Rapp E, Stahl B, Mearin ML, Koletzko B. Presence and Levels of Galactosyllactoses and Other Oligosaccharides in Human Milk and Their Variation during Lactation and According to Maternal Phenotype. Nutrients. 2021; 13(7):2324. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13072324
Chicago/Turabian StyleEussen, Simone R. B. M., Marko Mank, Robert Kottler, Xenia-Katharina Hoffmann, Alexander Behne, Erdmann Rapp, Bernd Stahl, M. Luisa Mearin, and Berthold Koletzko. 2021. "Presence and Levels of Galactosyllactoses and Other Oligosaccharides in Human Milk and Their Variation during Lactation and According to Maternal Phenotype" Nutrients 13, no. 7: 2324. https://0-doi-org.brum.beds.ac.uk/10.3390/nu13072324