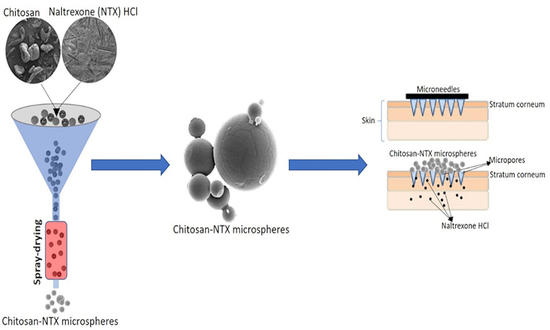
Design and Characterization of Spray-Dried Chitosan-Naltrexone Microspheres for Microneedle-Assisted Transdermal Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Design of Experiment (DoE)
2.3. Spray-Drying of Chitosan-NTX Dispersions
2.3.1. Chitosan-NTX Dispersion
2.3.2. Spray-Dried Chitosan-NTX Microspheres
2.4. Characterization of Chitosan-NTX Microspheres
2.4.1. Size Distribution and Morphology
2.4.2. Determination of NTX Content in Microspheres
2.4.3. Solid-State Characterization
2.5. In Vitro NTX Release Studies
2.6. In Vitro Skin Permeation Studies
2.6.1. Preparation of Chitosan-NTX Dispersions
2.6.2. Preparation of Porcine Ear Skin
2.6.3. In Vitro Skin Permeation
2.6.4. NTX Recovery from Porcine Skin
2.7. HPLC Method
2.8. Statistical Analysis
3. Results and Discussion
3.1. Quality Attributes and Characterization of Chitosan-NTX Microspheres
3.1.1. Summary of Quality Attributes
3.1.2. Influence of Parameters on Microspheres Quality Attributes
3.2. Shape and Surface Morphology
3.3. Solid State Characterization
3.3.1. FT-IR Spectra
3.3.2. DSC Thermograms
3.4. In Vitro Skin Permeation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Calcaterra, S.L.; Bach, P.; Chadi, A.; Chadi, N.; Kimmel, S.D.; Morford, K.L.; Roy, P.; Samet, J.H. Methadone Matters: What the United States Can Learn from the Global Effort to Treat Opioid Addiction. J. Gen. Intern. Med. 2019, 34, 1039–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudakin, D. Naltrexone: Not just for opioids anymore. J. Med Toxicol. 2016, 12, 71–75. [Google Scholar] [CrossRef]
- Banks, S.L.; Pinninti, R.R.; Gill, H.S.; Paudel, K.S.; Crooks, P.A.; Brogden, N.K.; Prausnitz, M.R.; Stinchcomb, A.L. Transdermal delivery of naltrexol and skin permeability lifetime after microneedle treatment in hairless guinea pigs. J. Pharm. Sci. 2010, 99, 3072–3080. [Google Scholar] [CrossRef] [Green Version]
- Wermeling, D.P.; Banks, S.L.; Hudson, D.A.; Gill, H.S.; Gupta, J.; Prausnitz, M.R.; Stinchcomb, A.L. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc. Natl. Acad. Sci. USA 2008, 105, 2058–2063. [Google Scholar] [CrossRef] [Green Version]
- Valiveti, S.; Paudel, K.S.; Hammell, D.C.; Hamad, M.O.; Chen, J.; Crooks, P.A.; Stinchcomb, A.L. In vitro/in vivo correlation of transdermal naltrexone prodrugs in hairless guinea pigs. Pharm. Res. 2005, 22, 981–989. [Google Scholar] [CrossRef]
- Milewski, M.; Paudel, K.S.; Brogden, N.K.; Ghosh, P.; Banks, S.L.; Hammell, D.C.; Stinchcomb, A.L. Microneedle-assisted percutaneous delivery of naltrexone hydrochloride in yucatan minipig: In vitro–in vivo correlation. J. Mol. Pharm. 2013, 10, 3745–3757. [Google Scholar]
- Brogden, N.K.; Banks, S.L.; Crofford, L.J.; Stinchcomb, A.L. Diclofenac enables unprecedented week-long microneedle-enhanced delivery of a skin impermeable medication in humans. Pharm. Res. 2013, 30, 1947–1955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalluri, H.; Banga, A.K. Transdermal delivery of proteins. Aaps Pharmscitech 2011, 12, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Singhvi, G.; Dubey, S.K.; Pandey, M.M.; Gupta, G.; Singh, M.; Dua, K. Microneedles: A smart approach and increasing potential for transdermal drug delivery system. Biomed. Pharmacother. 2019, 109, 1249–1258. [Google Scholar] [CrossRef]
- Banks, S.L.; Paudel, K.S.; Brogden, N.K.; Loftin, C.D.; Stinchcomb, A.L. Diclofenac enables prolonged delivery of naltrexone through microneedle-treated skin. Pharm. Res. 2011, 28, 1211–1219. [Google Scholar] [CrossRef] [Green Version]
- Srinivas, P.; Shanthi, C.L.; Sadanandam, M. Microneedle patches in drug delivery—A review. Int. J. Pharm. Technol. 2010, 2, 329–344. [Google Scholar]
- Martins, I.M.; Barreiro, M.F.; Coelho, M.; Rodrigues, A.E. Microencapsulation of essential oils with biodegradable polymeric carriers for cosmetic applications. Chem. Eng. J. 2014, 245, 191–200. [Google Scholar] [CrossRef] [Green Version]
- Tomaro-Duchesneau, C.; Saha, S.; Malhotra, M.; Kahouli, I.; Prakash, S. Microencapsulation for the therapeutic delivery of drugs, live mammalian and bacterial cells, and other biopharmaceutics: Current status and future directions. J. Pharm. 2012, 2013, 103527. [Google Scholar] [CrossRef] [PubMed]
- Ogunjimi, A.T.; Melo, S.M.; Vargas-Rechia, C.G.; Emery, F.S.; Lopez, R.F. Hydrophilic polymeric nanoparticles prepared from Delonix galactomannan with low cytotoxicity for ocular drug delivery. Carbohydr. Polym. 2017, 157, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Gelfuso, G.M.; Gratieri, T.; Simao, P.S.; de Freitas, L.A.P.; Lopez, R.F.V. Chitosan microparticles for sustaining the topical delivery of minoxidil sulphate. J. Microencapsul. 2011, 28, 650–658. [Google Scholar] [PubMed]
- Agnihotri, S.A.; Aminabhavi, T.M. Controlled release of clozapine through chitosan microparticles prepared by a novel method. J. Control Release 2004, 96, 245–259. [Google Scholar] [CrossRef]
- Peniche, C.; Argüelles-Monal, W.; Peniche, H.; Acosta, N. Chitosan: An attractive biocompatible polymer for microencapsulation. J. Macromol. Biosci. 2003, 3, 511–520. [Google Scholar] [CrossRef]
- Sosnik, A.; Seremeta, K.P. Advantages and challenges of the spray-drying technology for the production of pure drug particles and drug-loaded polymeric carriers. Adv. Colloid Interface Sci. 2015, 223, 40–54. [Google Scholar] [CrossRef]
- Santos, D.; Maurício, A.C.; Sencadas, V.; Santos, J.D.; Fernandes, M.H.; Gomes, P.S. Spray Drying: An Overview. In Biomaterials-Physics and Chemistry-New Edition; Intechopen: London, UK, 2017. [Google Scholar]
- Sun, Y.; Cui, F.; Shi, K.; Wang, J.; Niu, M.; Ma, R. The effect of chitosan molecular weight on the characteristics of spray-dried methotrexate-loaded chitosan microspheres for nasal administration. Drug Dev. Ind. Pharm. 2009, 35, 379–386. [Google Scholar]
- Xu, J.; Chen, Y.; Jiang, X.; Gui, Z.; Zhang, L. Development of Hydrophilic Drug Encapsulation and Controlled Release Using a Modified Nanoprecipitation Method. Processes 2019, 7, 331. [Google Scholar]
- El-Say, K.M. Maximizing the encapsulation efficiency and the bioavailability of controlled-release cetirizine microspheres using Draper-Lin small composite design. Drug Des. Dev. Ther. 2016, 10, 825–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommerfeld Ross, S.; Gharse, S.; Sanchez, L.; Fiegel, J. Dry powder aerosols to co-deliver antibiotics and nutrient dispersion compounds for enhanced bacterial biofilm eradication. Int. J. Pharm. 2017, 531, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Akerlof, G. Dielectric constants of some organic solvent-water mixtures at various temperatures. J. Am. Chem. Soc. 1932, 54, 4125–4139. [Google Scholar]
- Khattab, I.S.; Bandarkar, F.; Fakhree, M.A.A.; Jouyban, A. Density, viscosity, and surface tension of water+ ethanol mixtures from 293 to 323K. Korean J. Chem. Eng. 2012, 29, 812–817. [Google Scholar]
- Riobóo, R.J.; Philipp, M.; Ramos, M.; Krüger, J.-K. Concentration and temperature dependence of the refractive index of ethanol-water mixtures: Influence of intermolecular interactions. Eur. Phys. J. E 2009, 30, 19. [Google Scholar]
- Rasband, W.S. ImageJ; US National Institutes of Health: Bethesda, MD, USA, 2011–2018. Available online: http://imagej.nih.gov/ij/ (accessed on 27 May 2020).
- Prota, L.; Santoro, A.; Bifulco, M.; Aquino, R.P.; Mencherini, T.; Russo, P. Leucine enhances aerosol performance of Naringin dry powder and its activity on cystic fibrosis airway epithelial cells. Int. J. Pharm. 2011, 412, 8–19. [Google Scholar] [CrossRef]
- Tewa-Tagne, P.; Degobert, G.; Briançon, S.; Bordes, C.; Gauvrit, J.-Y.; Lanteri, P.; Fessi, H. Spray-drying Nanocapsules in Presence of Colloidal Silica as Drying Auxiliary Agent: Formulation and Process Variables Optimization Using Experimental Designs. Pharm. Res. 2007, 24, 650–661. [Google Scholar] [CrossRef]
- Habtegebriel, H.; Wawire, M.; Sila, D. The Effect of Pretreatment (Spray Drying) on the Yield and Selected Nutritional Components of Whole Camel Milk Powder. J. Food Sci. 2018, 83, 2983–2991. [Google Scholar] [CrossRef]
- LeClair, D.A.; Cranston, E.D.; Xing, Z.; Thompson, M.R. Optimization of spray drying conditions for yield, particle size and biological activity of thermally stable viral vectors. Pharm. Res. 2016, 33, 2763–2776. [Google Scholar] [CrossRef] [Green Version]
- Maury, M.; Murphy, K.; Kumar, S.; Shi, L.; Lee, G. Effects of process variables on the powder yield of spray-dried trehalose on a laboratory spray-dryer. Eur. J. Pharm. Biopharm. 2005, 59, 565–573. [Google Scholar] [CrossRef]
- Billon, A.; Bataille, B.; Cassanas, G.; Jacob, M. Development of spray-dried acetaminophen microparticles using experimental designs. Int. J. Pharm. 2000, 203, 159–168. [Google Scholar] [CrossRef]
- Zhang, T.; Youan, B.-B.C. Analysis of process parameters affecting spray-dried oily core nanocapsules using factorial design. Aaps Pharmscitech 2010, 11, 1422–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, K.G.H.; Park, H.J. Preparation and characterization of drug-loaded chitosan–tripolyphosphate microspheres by spray drying. Drug Dev. Res. 2005, 64, 114–128. [Google Scholar] [CrossRef]
- Aranaz, I.; Paños, I.; Peniche, C.; Heras, Á.; Acosta, N. Chitosan Spray-Dried Microparticles for Controlled Delivery of Venlafaxine Hydrochloride. Molecules 2017, 22, 1980. [Google Scholar] [CrossRef] [Green Version]
- Fazaeli, M.; Emam-Djomeh, Z.; Kalbasi Ashtari, A.; Omid, M. Effect of spray drying conditions and feed composition on the physical properties of black mulberry juice powder. Food Bioprod. Process. 2012, 90, 667–675. [Google Scholar] [CrossRef]
- Schmitz-Schug, I.; Foerst, P.; Kulozik, U. Impact of the spray drying conditions and residence time distribution on lysine loss in spray dried infant formula. Dairy Sci. Technol. 2013, 93, 443–462. [Google Scholar] [CrossRef] [Green Version]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of process conditions on the physicochemical properties of açai (Euterpe oleraceae Mart.) powder produced by spray drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Gianfrancesco, A.; Turchiuli, C.; Dumoulin, E.; Palzer, S. Prediction of powder stickiness along spray drying process in relation to agglomeration. Part. Sci. Technol. 2009, 27, 415–427. [Google Scholar] [CrossRef]
- Elversson, J.; Millqvist-Fureby, A. Particle Size and Density in Spray Drying—Effects of Carbohydrate Properties. J. Pharm. Sci. 2005, 94, 2049–2060. [Google Scholar] [CrossRef]
- Pavanetto, F.; Genta, I.; Giunchedi, P.; Conti, B.; Conte, U. Spray-dried albumin microspheres for the intra-articular delivery of dexamethasone. J. Microencapsul. 1994, 11, 445–454. [Google Scholar] [CrossRef]
- Kosaraju, S.L.; D’Ath, L.; Lawrence, A. Preparation and characterisation of chitosan microspheres for antioxidant delivery. Carbohydr. Polym. 2006, 64, 163–167. [Google Scholar] [CrossRef]
- Desai, K.; Park, H.J. Effect of manufacturing parameters on the characteristics of vitamin C encapsulated tripolyphosphate-chitosan microspheres prepared by spray-drying. J. Microencapsul. 2006, 23, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Jabrail, F.H. Glutaraldehyde cross-linked chitosan microspheres for controlled release of centchroman. Carbohydr. Res. 2007, 342, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Chiang, C.H.; Yeh, M.K. Optimizing formulation factors in preparing chitosan microparticles by spray-drying method. J. Microencapsul. 2003, 20, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Learoyd, T.P.; Burrows, J.L.; French, E.; Seville, P.C. Chitosan-based spray-dried respirable powders for sustained delivery of terbutaline sulfate. Eur. J. Pharm. Biopharm. 2008, 68, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Moffat, A.C.; Osselton, M.D.; Widdop, B.; Watts, J. Naltrexone. In Clarke’s Analysis of Drugs and Poisons; Galichet, L.Y., Moffat, A.C., Osselton, M.D., Widdop, B., Eds.; Pharmaceutical Press London: London, UK, 2011; Volume 3. [Google Scholar]
- Song, C.; Yu, H.; Zhang, M.; Yang, Y.; Zhang, G. Physicochemical properties and antioxidant activity of chitosan from the blowfly Chrysomya megacephala larvae. Int. J. Biol. Macromol. 2013, 60, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, G.; Miranda, S.P. FTIR and TGA studies of chitosan composite films. J. Chil. Chem. Soc. 2004, 49, 291–295. [Google Scholar] [CrossRef]
- Altınışık, A.; Seki, Y.; Yurdakoc, K. Preparation and characterization of chitosan/KSF biocomposite film. Polym. Compos. 2009, 30, 1035–1042. [Google Scholar] [CrossRef]






| Run | A | B | C | D | Yield (%) | Mean Diameter (µm) | Mean zeta Potential (mV) | EE (%) | Drug-Loading Capacity (%) | Outlet Air Temperature (°C) | NTX Release (%) | Dry Batch Weight (g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F1 | 0 | −1 | 0 | −1 | 38.6 | 2.69 ± 0.35 | 52.95 ± 2.62 | 78.0 ± 0.2 | 15.6 ± 0.04 | 84 | 69.4 ± 4.4 | 1.5 |
| F2 | 0 | 0 | 0 | 0 | 23.4 | 2.03 ± 0.39 | 59.93 ± 6.05 | 84.2 ± 0.3 | 16.8 ± 0.07 | 73 | 74.7 ± 1.1 | 1.5 |
| F3 | 1 | 0 | 0 | −1 | 33.2 | 1.73 ± 0.13 | 55.33 ± 1.80 | 86.5 ± 0.5 | 17.3 ± 0.10 | 87 | 65.1 ± 3.9 | 1.5 |
| F4 | 1 | 1 | 0 | 0 | 39.0 | 2.25 ± 0.15 | 60.37 ± 6.93 | 86.1 ± 4.5 | 17.2 ± 0.91 | 80 | 74.9 ± 20.6 | 2 |
| F5 | 0 | 0 | −1 | −1 | 33.7 | 8.35 ± 0.41 | 42.03 ± 5.22 | 80.1 ± 1.1 | 40.0 ± 0.55 | 88 | 81.5 ± 7.1 | 1.4 |
| F6 | 1 | 0 | 1 | 0 | 29.4 | 3.34 ± 0.19 | 47.45 ± 7.14 | 88.3 ± 3.3 | 12.6 ± 0.47 | 76 | 65.8 ± 4.0 | 1.4 |
| F7 | 1 | 0 | −1 | 0 | 35.4 | 7.31 ± 0.89 | 34.23 ± 1.35 | 85.1 ± 1.4 | 42.5 ± 0.72 | 80 | 72.1 ± 4.2 | 2 |
| F8 | 0 | −1 | 0 | 1 | 29.7 | 2.84 ± 0.10 | 59.70 ± 6.30 | 73.6 ± 0.6 | 14.7 ± 0.11 | 60 | 67.2 ± 0.6 | 1.5 |
| F9 | 0 | 0 | 0 | 0 | 33.1 | 2.71 ± 0.23 | 60.75 ± 0.21 | 73.8 ± 2.6 | 14.8 ± 0.52 | 85 | 81.4 ± 0.8 | 1.5 |
| F10 | 0 | −1 | −1 | 0 | 31.6 | 9.20 ± 1.33 | 43.67 ± 0.46 | 71.0 ± 0.0 | 35.5 ± 0.00 | 75 | 79.7 ± 3.4 | 1.4 |
| F11 | 0 | 0 | 1 | 1 | 30.0 | 3.21 ± 0.22 | 61.67 ± 5.23 | 70.5 ± 0.3 | 10.1 ± 0.05 | 60 | 79.8 ± 1.1 | 1.4 |
| F12 | 0 | 0 | 1 | −1 | 33.6 | 2.93 ± 0.18 | 59.47 ± 1.68 | 69.8 ± 1.7 | 10.0 ± 0.24 | 98 | 82.9 ± 6.2 | 1.4 |
| F13 | 0 | −1 | 1 | 0 | 34.5 | 2.08 ± 0.15 | 44.40 ± 5.26 | 70.2 ± 3.2 | 10.1 ± 0.46 | 89 | 86.5 ± 3.1 | 1.87 |
| F14 | −1 | 0 | 0 | 1 | 25.4 | 3.84 ± 0.20 | 61.33 ± 0.91 | 72.3 ± 1.9 | 14.5 ± 0.39 | 60 | 88.6 ± 6.6 | 1.5 |
| F15 | 1 | 0 | 0 | 1 | 17.0 | 2.48 ± 0.15 | 57.05 ± 2.47 | 73.7 ± 0.1 | 14.7 ± 0.02 | 63 | 71.5 ± 1.4 | 1.5 |
| F16 | −1 | 0 | 0 | −1 | 40.3 | 2.64 ± 0.16 | 66.50 ± 0.36 | 74.9 ± 3.5 | 15.0 ± 0.70 | 83 | 74.8 ± 6.1 | 1.5 |
| F17 | 1 | −1 | 0 | 0 | 32.2 | 3.23 ± 0.11 | 63.20 ± 2.17 | 80.9 ± 2.8 | 16.2 ± 0.55 | 75 | 80.7 ± 3.2 | 1.5 |
| F18 | 0 | 1 | −1 | 0 | 41.0 | 3.67 ± 0.23 | 56.23 ± 7.57 | 85.1 ± 0.3 | 42.6 ± 0.17 | 80 | 74.0 ± 4.8 | 1.4 |
| F19 | 0 | 0 | −1 | 1 | 20.7 | 3.67 ± 0.13 | 46.90 ± 2.51 | 77.5 ± 1.9 | 38.8 ± 0.95 | 61 | 94.0 ± 4.2 | 1.6 |
| F20 | −1 | −1 | 0 | 0 | 37.9 | 2.19 ± 0.33 | 57.10 ± 1.41 | 75.5 ± 1.9 | 15.1 ± 0.38 | 81 | 78.8 ± 13.2 | 1.5 |
| F21 | 0 | 0 | 0 | 0 | 35.6 | 2.98 ± 0.08 | 61.30 ± 2.97 | 81.2 ± 1.7 | 16.2 ± 0.33 | 82 | 76.2 ± 5.6 | 2 |
| F22 | 0 | 1 | 0 | 1 | 32.7 | 2.71 ± 0.21 | 57.40 ± 7.38 | 80.9 ± 3.2 | 16.2 ± 0.64 | 61 | 74.0 ± 5.0 | 1.5 |
| F23 | −1 | 1 | 0 | 0 | 29.0 | 2.92 ± 0.24 | 63.03 ± 5.24 | 76.7 ± 4.7 | 15.3 ± 0.94 | 85 | 82.0 ± 9.3 | 2 |
| F24 | 0 | 1 | 0 | −1 | 39.5 | 3.14 ± 0.09 | 63.40 ± 0.71 | 75.5 ± 3.9 | 15.1 ± 0.79 | 86 | 78.2 ± 2.0 | 1.5 |
| F25 | −1 | 0 | 1 | 0 | 43.2 | 2.20 ± 0.21 | 57.73 ± 2.50 | 77.0 ± 1.6 | 11.0 ± 0.22 | 79 | 72.7 ± 6.3 | 1.4 |
| F26 | 0 | 1 | 1 | 0 | 38.9 | 2.52 ± 0.27 | 56.47 ± 4.25 | 81.5 ± 4.3 | 11.6 ± 0.61 | 81 | 62.9 ± 11.8 | 1.4 |
| F27 | −1 | 0 | −1 | 0 | 23.4 | 8.29 ± 0.70 | 52.17 ± 0.21 | 74.9 ± 4.8 | 37.5 ± 2.42 | 70 | 89.0 ± 1.5 | 2 |
| Coded Variables | Uncoded Variables | −1 | 0 | 1 |
|---|---|---|---|---|
| A | Chitosan molecular weight | L | M | H |
| B | Chitosan concentration (%w/v) | 0.5 | 0.7 | 0.9 |
| C | Chitosan-NTX ratio | 1 | 3 | 5 |
| D | Feed flow rate (mL/min) | 4 | 6 | 8 |
| Individual Effects on | |||||||
|---|---|---|---|---|---|---|---|
| Input Variables | Yield (%) | Diameter (µm) | Zeta Potential (mV) | Outlet Air Temperature (°C) | EE (%) | Drug-Loading Capacity (%) | NTX Release (%) |
| Chitosan MW | p = 0.476 | p = 0.718 | p = 0.975 | p = 0.877 | p = 0.009 * | p = 0.579 | p = 0.043 * |
| Chitosan concentration | p = 0.394 | p = 0.372 | p = 0.352 | p = 0.643 | p = 0.042 * | p = 0.626 | p = 0.532 |
| Chitosan-NTX ratio | p = 0.207 | p = 0.001 * | p = 0.113 | p = 0.147 | p = 0.335 | p < 0.0001* | p = 0.138 |
| Feed flow rate | p = 0.003 * | p = 0.673 | p = 0.618 | p < 0.001 * | p = 0.341 | p = 0.854 | p = 0.373 |
| Formulations | Chitosan MW (kDa) | Yield (%) | Mean Diameter (µm) | Encapsulation Efficiency (%) | Drug-Loading Capacity (%) | Cumulative in Vitro NTX Release (%) | * NTX Flux in MN-Treated Porcine Skin (µg/cm2·h) |
|---|---|---|---|---|---|---|---|
| F7 | 310–375 | 35.4 | 7.31 ± 0.89 | 85.1 ± 1.4 | 42.5 ± 0.72 | 72.1 ± 4.2 | 3.89 ± 0.96 |
| F19 | 190–310 | 20.7 | 3.67 ± 0.13 | 77.5 ± 1.9 | 38.8 ± 0.95 | 94.0 ± 4.2 | 4.24 ± 1.57 |
| F27 | 50–190 | 23.4 | 8.29 ± 0.70 | 74.9 ± 4.8 | 37.5 ± 2.42 | 89.0 ± 1.5 | 3.28 ± 0.98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogunjimi, A.T.; Fiegel, J.; Brogden, N.K. Design and Characterization of Spray-Dried Chitosan-Naltrexone Microspheres for Microneedle-Assisted Transdermal Delivery. Pharmaceutics 2020, 12, 496. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060496
Ogunjimi AT, Fiegel J, Brogden NK. Design and Characterization of Spray-Dried Chitosan-Naltrexone Microspheres for Microneedle-Assisted Transdermal Delivery. Pharmaceutics. 2020; 12(6):496. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060496
Chicago/Turabian StyleOgunjimi, Abayomi T., Jennifer Fiegel, and Nicole K. Brogden. 2020. "Design and Characterization of Spray-Dried Chitosan-Naltrexone Microspheres for Microneedle-Assisted Transdermal Delivery" Pharmaceutics 12, no. 6: 496. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics12060496