Electrospun Poly(lactide) Fibers as Carriers for Controlled Release of Biochanin A
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Solutions for Electrospinning
2.3. Stretching of the Material
2.4. Determination of the Wetting Angle
2.5. Differential Scanning Calorimetry (DSC)
2.6. Fourier Transform Jnfrared Spectroscopy (FTIR)
2.7. Scanning Electron Microscopy (SEM)
2.8. Modified Release of Biochanin A from Electrospun PLA Fibers
2.9. High Performance Liquid Chromatography (HPLC)
2.10. Cell Proliferation Assay
2.11. In Vitro Wound Healing Assay
2.12. Statistical Analysis
3. Results
3.1. Preparation and Characterization of Electrospun PLA Fibers with and without Biochanin A
3.1.1. Mechanical Properties of Electrospun PLA Fibers
3.1.2. Surface Properties of Electrospun PLA Fibers
3.1.3. Thermal Properties of Electrospun PLA Fibers
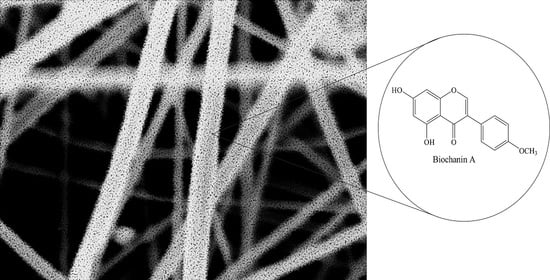
3.1.4. Morphology of the Electrospun PLA Fibers
3.1.5. FTIR Analysis
3.2. Modified Release of Biochanin A from Electrospun PLA Fibers
3.3. Biological Testing
3.3.1. Cell Proliferation
3.3.2. In Vitro Wound Healing Activity
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arshad, R.; Tabish, T.; Kiani, M.H.; Ibrahim, I.; Shahnaz, G.; Rahdar, A.; Kang, M.; Pandey, S. A Hyaluronic Acid Functionalized Self-Nano-Emulsifying Drug Delivery System (SNEDDS) for Enhancement in Ciprofloxacin Targeted Delivery against Intracellular Infection. Nanomaterials 2021, 11, 1086. [Google Scholar] [CrossRef] [PubMed]
- Sargazi, S.; Hajinezhad, M.R.; Barani, M.; Rahdar, A.; Shahraki, S.; Karimi, P.; Cucchiarini, M.; Khatami, M.; Pandey, S. Synthesis, characterization, toxicity and morphology assessments of newly prepared microemulsion systems for delivery of valproic acid. J. Mol. Liq. 2021, 338, 116625. [Google Scholar] [CrossRef]
- Mali, R.R.; Goel, V.; Gupta, S. Novel study in sustained release drug delivery system: A Review. Int. J. Pharm. Med. Res. 2015, 3, 204–215. [Google Scholar]
- Sahoo, S.K.; Parveen, S.; Panda, J.J. The present and future of nanotechnology in human health care. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.N.; Xu, X.; Wen, N.; Song, R.; Meng, Q.; Guan, Y.; Cheng, S.; Cao, D.; Dong, Y.; Qie, J.; et al. A drug carrier for sustained zero-order release of peptide therapeutics. Sci. Rep. 2017, 7, 5524. [Google Scholar] [CrossRef] [Green Version]
- Fina, F.; Goyanes, A.; Rowland, M.; Gaisford, S.; Basit, A.W. 3D printing of tunable zero-order release printlets. Polymers 2020, 12, 1769. [Google Scholar] [CrossRef]
- Li, X.; Li, Q.; Zhao, C. Zero-Order Controlled Release of Water-Soluble Drugs Using a Marker Pen Platform. ACS Omega 2021, 6, 13774–13778. [Google Scholar] [CrossRef]
- Leach, M.; Feng, Z.Q.; Tuck, S.; Corey, J. Electrospinning Fundamentals: Optimizing Solution and Apparatus Parameters. JoVE 2011, 47, 2494. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, S.; Lu, W.; Wang, Y.; Zhang, P.; Yao, Q. Electrospun fibers and their application in drug controlled release, biological dressings, tissue repair, and enzyme immobilization. RSC Adv. 2019, 9, 25712–25729. [Google Scholar] [CrossRef] [Green Version]
- Torres-Martínez, E.J.; Cornejo Bravo, J.M.; Serrano Medina, A.; Pérez González, G.L.; Villarreal Gómez, L.J. A summary of electrospun nanofibers as drug delivery system: Drugs loaded and biopolymers used as matrices. Curr. Drug Deliv. 2018, 15, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Wang, Y.; Tong, A.; Guo, G. Facile electrospinning of an efficient drug delivery system. Expert Opin. Drug Deliv. 2016, 13, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, J.; Cheng, H.; Li, G.; Cho, H.; Jiang, M.; Gao, Q.; Zhang, X. Developments of Advanced Electrospinning Techniques: A Critical Review. Adv. Mater. Technol. 2021, 6, 2100410. [Google Scholar] [CrossRef]
- Lasprilla, A.J.; Martinez, G.A.; Lunelli, B.H.; Jardini, A.L.; Maciel Filho, R. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- Raquez, J.M.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA)-based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Cui, S.; Sun, X.; Li, K.; Gou, D.; Zhou, Y.; Hu, J.; Liu, Y. Polylactide nanofibers delivering doxycycline for chronic wound treatment. Mater. Sci. Eng. C 2019, 104, 109745. [Google Scholar] [CrossRef]
- Huang, C.; Soenen, S.; Gulck, E.; Vanham, G.; Rejman, J.; Calenbergh, S.; Vervaet, C.; Coenye, T.; Verstraelen, H.; Temmerman, M.; et al. Electrospun cellulose acetate phthalate fibers for semen induced anti-HIV vaginal drug delivery. Biomaterials 2012, 33, 962–969. [Google Scholar] [CrossRef]
- Shrestha, R.; Palat, A.; Punnoose, A.; Joshi, S.; Ponraju, D.; Paul, S. Electrospun cellulose acetate phthalate nanofibrous scaffoldsfabricated using novel solvent combinations biocompatible forprimary chondrocytes and neurons. Tissue Cell. 2016, 48, 634–643. [Google Scholar] [CrossRef]
- Hua, D.; Liu, Z.; Wang, F.; Gao, B.; Chen, F.; Zhang, Q.; Xiong, R.; Han, J.; Samal, S.; De Smedt, S.; et al. pH responsive polyurethane (core) and cellulose acetate phthalate (shell) electrospun fibers for intravaginal drug delivery. Carbohydr. Polym. 2016, 151, 1240–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vidal-Romero, G.; Rocha-Pérez, V.; Zambrano-Zaragoza, M.; Del Real, A.; Martínez-Acevedo, L.; Galindo-Pérez, M.; Quintanar-Guerrero, D. Development and Characterization of pH-Dependent Cellulose Acetate Phthalate Nanofibers by Electrospinning Technique. Nanomaterials 2021, 11, 3202. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Li, X.; Zhu, X.; Yu, G.; Zhou, S.; Weng, J. Investigation of drug release and matrix degradation of electrospun poly(DL-lactide) fibers with paracetamol inoculation. Biomacromolecules 2006, 7, 1623–1629. [Google Scholar] [CrossRef]
- Valenti, S.; Del Valle, L.; Yousefzade, O.; Macovez, R.; Franco, L.; Puiggalí, J. Chloramphenicol loaded polylactide melt electrospun scaffolds for biomedical applications. Int. J. Pharm. 2021, 606, 120897. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, X.; Zhang, Z.; Zhang, Y.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Use of asymmetric multilayer polylactide nanofiber mats in controlled release of drugs and prevention of liver cancer recurrence after surgery in mice. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, P.; Lou, L.; Wang, Y. Perspectives regarding the role of biochanin A in humans. Front. Pharmacol. 2019, 10, 793. [Google Scholar] [CrossRef] [Green Version]
- Sarfraz, A.; Javeed, M.; Shah, M.A.; Hussain, G.; Shafiq, N.; Sarfraz, I.; Rasul, A. Biochanin A: A novel bioactive multifunctional compound from nature. Sci. Total Environ. 2020, 722, 137907. [Google Scholar] [CrossRef]
- Raheja, S.; Girdhar, A.; Lather, V.; Pandita, D. Biochanin A: A phytoestrogen with therapeutic potential. Trends Food Sci. Technol. 2018, 79, 55–66. [Google Scholar] [CrossRef]
- Oseni, T.; Patel, R.; Pyle, J.; Jordan, V.C. Selective estrogen receptor modulators and phytoestrogens. Planta Med. 2008, 74, 1656–1665. [Google Scholar] [CrossRef] [Green Version]
- Vitale, D.C.; Piazza, C.; Melilli, B.; Drago, F.; Salomone, S. Isoflavones: Estrogenic activity, biological effect and bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 15–25. [Google Scholar] [CrossRef]
- Tanwar, A.K.; Dhiman, N.; Kumar, A.; Jaitak, V. Engagement of phytoestrogens in breast cancer suppression: Structural classification and mechanistic approach. Eur. J. Med. Chem. 2021, 213, 113037. [Google Scholar] [CrossRef]
- Domínguez-López, I.; Yago-Aragón, M.; Salas-Huetos, A.; Tresserra-Rimbau, A.; Hurtado-Barroso, S. Effects of dietary phytoestrogens on hormones throughout a human lifespan: A review. Nutrients 2020, 12, 2456. [Google Scholar] [CrossRef] [PubMed]
- Kole, L.; Giri, B.; Manna, S.K.; Pal, B.; Ghosh, S. Biochanin-A, an isoflavon, showed anti-proliferative and anti-inflammatory activities through the inhibition of iNOS expression, p38-MAPK and ATF-2 phosphorylation and blocking NFκB nuclear translocation. Eur. J. Pharmacol. 2011, 653, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Medjakovic, S.; Jungbauer, A. Red clover isoflavones biochanin A and formononetin are potent ligands of the human aryl hydrocarbon receptor. J. Steroid Biochem. Mol. Biol. 2008, 108, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Han, E.H.; Kim, J.Y.; Jeong, H.G. Effect of biochanin A on the aryl hydrocarbon receptor and cytochrome P450 1A1 in MCF-7 human breast carcinoma cells. Arch. Pharm. Res. 2006, 29, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.G.; Kim, J.E.; Jung, S.K.; Li, Y.; Bode, A.M.; Park, J.S.; Lee, K.W. MLK3 is a direct target of biochanin A, which plays a role in solar UV-induced COX-2 expression in human keratinocytes. Biochem. Pharmacol. 2013, 86, 896–903. [Google Scholar] [CrossRef] [Green Version]
- Hollmerus, S.; Yousaf, S.S.; Islam, Y.; Khan, I. Isoflavones-Based Liposome Formulations as Anti-Aging for Skincare. Nov. Approaches Drug Des. Dev. 2018, 3, 555615. [Google Scholar]
- Kalayciyan, A.; Orawa, H.; Fimmel, S.; Perschel, F.H.; González, J.B.; Fitzner, R.G.; Zouboulis, C.C. Nicotine and biochanin A, but not cigarette smoke, induce anti-inflammatory effects on keratinocytes and endothelial cells in patients with Behcet’s disease. J. Investig. Dermatol. 2007, 127, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Lin, V.C.; Ding, H.Y.; Tsai, P.C.; Wu, J.Y.; Lu, Y.H.; Chang, T.S. In vitro and in vivo melanogenesis inhibition by biochanin A from Trifolium pratense. Biosci. Biotechnol. Biochem. 2011, 75, 914–918. [Google Scholar] [CrossRef] [Green Version]
- Lueangarun, S.; Panchaprateep, R. An Herbal Extract Combination (Biochanin A, Acetyl tetrapeptide-3, and Ginseng Extracts) versus 3% Minoxidil Solution for the Treatment of Androgenetic Alopecia: A 24-week, Prospective, Randomized, Triple-blind, Controlled Trial. J. Clin. Aesthet. Dermatol. 2020, 13, 32–37. [Google Scholar]
- Hajrezaie, M.; Salehen, N.; Karimian, H.; Zahedifard, M.; Shams, K.; Batran, R.A.; Abdulla, M.A. Biochanin a gastroprotective effects in ethanol-induced gastric mucosal ulceration in rats. PLoS ONE 2015, 10, e0121529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breikaa, R.M.; Algandaby, M.M.; El-Demerdash, E.; Abdel-Naim, A.B. Biochanin A protects against acute carbon tetrachloride-induced hepatotoxicity in rats. Biosci. Biotechnol. Biochem. 2013, 77, 909–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govindasami, S.; Uddandrao, V.V.; Raveendran, N.; Sasikumar, V. Therapeutic potential of biochanin-A against isoproterenol-induced myocardial infarction in rats. Cardiovasc. Hematol. Agents Med. Chem. 2020, 18, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Govindasami, S.; Uddandrao, V.V.; Jansy Isabella, R.A.R.; Saravanan, G.; Ponmurugan, P.; Chandrasekaran, P.; Vadivukkarasi, S. Attenuation of lipid metabolic abnormalities, proinflammatory cytokines, and matrix metalloproteinase expression by biochanin-A in isoproterenol-induced myocardial infarction in rats. Drug Chem. Toxicol. 2021, 1–12. [Google Scholar] [CrossRef]
- Guo, M.; Lu, H.; Qin, J.; Qu, S.; Wang, W.; Guo, Y.; Wang, Y. Biochanin A provides neuroprotection against cerebral ischemia/reperfusion injury by Nrf2-mediated inhibition of oxidative stress and inflammation signaling pathway in rats. Med. Sci. Monit. 2019, 25, 8975–8983. [Google Scholar] [CrossRef] [PubMed]
- Oza, M.J.; Kulkarni, Y.A. Biochanin A improves insulin sensitivity and controls hyperglycemia in type 2 diabetes. Biomed. Pharmacother. 2018, 107, 1119–1127. [Google Scholar] [CrossRef]
- Singh, G.; Thaker, R.; Sharma, A.; Parmar, D. Therapeutic effects of biochanin A, phloretin, and epigallocatechin-3-gallate in reducing oxidative stress in arsenic-intoxicated mice. Environ. Sci. Pollut. Res. 2021, 28, 20517–20536. [Google Scholar] [CrossRef]
- Jalaludeen, A.M.; Ha, W.T.; Lee, R.; Kim, J.H.; Do, J.T.; Park, C.; Song, H. Biochanin A ameliorates arsenic-induced hepato-and hematotoxicity in rats. Molecules 2016, 21, 69. [Google Scholar] [CrossRef] [Green Version]
- Wahby, M.M.; Mohammed, D.S.; Newairy, A.A.; Abdou, H.M.; Zaky, A. Aluminum-induced molecular neurodegeneration: The protective role of genistein and chickpea extract. Food Chem. Toxicol. 2017, 107, 57–67. [Google Scholar] [CrossRef]
- Moon, Y.J.; Sagawa, K.; Frederick, K.; Zhang, S.; Morris, M.E. Pharmacokinetics and bioavailability of the isoflavone biochanin A in rats. AAPS J. 2006, 8, E433–E442. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, I.; Savić, I.; Popsavin, M.; Rakić, S.; Mihajilov-Krstev, T.; Ristić, I.; Erić, S.; Savić-Gajić, I. Preparation, characterization and antimicrobial activity of inclusion complex of biochanin A with (2-hydroxypropyl)-β-cyclodextrin. J. Pharm. Pharmacol. 2018, 70, 1485–1493. [Google Scholar] [CrossRef]
- Gajić, I.; Ilić-Stojanović, S.; Dinić, A.; Zdravković, A.; Stanojević, Lj.; Nikolić, V.; Nikolić, Lj. Modified Biochanin A Release from Dual pH and Thermo-Responsive Copolymer Hydrogels. Polymers 2021, 13, 426. [Google Scholar] [CrossRef] [PubMed]
- Hanski, L.; Genina, N.; Uvell, H.; Malinovskaja, K.; Gylfe, A.; Laaksonen, T.; Kolakovic, R.; Makila, E.; Salonen, J.; Hirvonen, J.; et al. Inhibitory Activity of the Isoflavone Biochanin A on Intracellular Bacteria of Genus Chlamydia and Initial Development of a Buccal Formulation. PLoS ONE 2014, 9, e115115. [Google Scholar] [CrossRef] [PubMed]
- Ristić, I.; Miletić, A.; Vukić, N.; Marinović-Cincović, M.; Smits, K.; Cakić, S.; Pilić, B. Characterization of electrospun poly(lactide) composites containing multiwalled carbon nanotubes. J. Thermoplast. Compos. Mater. 2021, 34, 695–706. [Google Scholar] [CrossRef]
- Radusin, T.; Torres-Giner, S.; Stupar, A.; Ristic, I.; Miletic, A.; Novakovic, A.; Lagaron, J.M. Preparation, characterization and antimicrobial properties of electrospun polylactide films containing Allium ursinum L. extract. Food Packag. Shelf Life. 2019, 21, 100357. [Google Scholar] [CrossRef]
- Miletic, A.; Pavlic, B.; Ristic, I.; Zekovic, Z.; Pilic, B. Encapsulation of Fatty Oils into Electrospun Nanofibers for Cosmetic Products with Antioxidant Activity. Appl. Sci. 2019, 9, 2955. [Google Scholar] [CrossRef] [Green Version]
- Savić, V.; Nikolić, V.; Arsić, I.; Stanojević, L.; Najman, S.; Stojanović, S.; Mladenović-Ranisavljević, I. Comparative Study of the Biological Activity of Allantoin and Aqueous Extract of the Comfrey Root. Phytother Res. 2015, 29, 1117–1122. [Google Scholar] [CrossRef]
- Stojanović, S.; Najman, S. The Effect of Conditioned Media of Stem Cells Derived from Lipoma and Adipose Tissue on Macrophages’ Response and Wound Healing in Indirect Co-culture System In Vitro. Int. J. Mol. Sci. 2019, 20, 1671. [Google Scholar] [CrossRef] [Green Version]
- Tasić-Kostov, M.; Arsić, I.; Pavlović, D.; Stojanović, S.; Najman, S.; Naumović, S.; Tadić, V. Towards a modern approach to traditional use: In vitro and in vivo evaluation of Alchemilla vulgaris L. gel wound healing potential. J. Ethnopharmacol. 2019, 238, 111789. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Đorđević, S.; Isailović, T.; Cekić, N.; Vuleta, G.; Savić, S. Diazepam-loaded parenteral nanoemulsions: Physicochemical characterization and in vitro release study. Arh. Farm. 2016, 66, 24–41. [Google Scholar]
- Zhang, Y.; Huo, M.; Zhou, J.; Zou, A.; Li, W.; Yao, C.; Xie, S. DDSolver: An add-in program for modeling and comparison of drug dissolution profiles. AAPS J. 2010, 12, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, P.; Gupta, P.; Srivastava, A.K.; Poluri, K.M.; Prasad, R. Eucalyptol/β-cyclodextrin inclusion complex loaded gellan/PVA nanofibers as antifungal drug delivery system. Int. J. Pharm. 2021, 609, 121163. [Google Scholar] [CrossRef] [PubMed]









| Sample Name | Electrospinning Process Parameters | ||
|---|---|---|---|
| Flow Rate (cm3/h) | Needle-to-Collector Distance (cm) | Voltage (kV) | |
| PLA | 2.5 | 10 | 14 |
| PLA-BCA-2% | 3 | 10 | 13 |
| PLA-BCA-5% | 3 | 10 | 14 |
| Sample Name | Max Stress (N/mm2) | Max Stroke-Strain (%) | Break Stress (N/mm2) | Break Stroke-Strain (%) |
|---|---|---|---|---|
| PLA | 0.44 | 4.69 | 0.53 | 5.47 |
| PLA-BCA-2% | 0.566 | 4.42 | 0.22 | 6.78 |
| PLA-BCA-5% | 2.083 | 4.00 | 0.35 | 37.6 |
| Sample Name | Contact Angle (°) |
|---|---|
| PLA | 101.53 |
| PLA-BCA-2% | 118.13 |
| PLA-BCA-5% | 126.63 |
| Vibration | Peak in the Spectrum of PLA, cm−1 | Peak in the Spectrum of PLA-BCA-5%, cm−1 |
|---|---|---|
| νs(C-H) | 2853 | 2851 |
| νas(C-H) | 2945 | 2946 |
| νs(C-H) from CH3 | 2880 | 2881 |
| νas(C-H) from CH3 | 2996 | 2996 |
| νs(C-O-C) | 1089 | 1089 |
| νas(C-O-C) | 1184 | 1184 |
| ν(C=O) | 1758 | 1759 |
| Kinetic Model | Parameter | PLA-BCA-2% | PLA-BCA-5% |
|---|---|---|---|
| Zero Order | k0 R2adj. AIC MSC | 0.245 0.9089 100.85 2.19 | 0.118 0.9282 93.35 2.47 |
| First Order | k1 R2adj. AIC MSC | 0.003 0.9237 97.66 2.37 | 0.001 0.9499 86.87 2.83 |
| Korsmeyer-Peppas | kKP n R2adj. AIC MSC | 1.157 0.679 0.9456 92.48 2.66 | 0.696 0.678 0.9718 77.44 3.35 |
| Baker-Lonsdale | kBL R2adj. AIC MSC | 0 0.9170 99.17 2.28 | 0 0.9436 88.99 2.71 |
| Higuchi | kH R2adj. AIC MSC | 2.654 0.9285 96.50 2.43 | 1.779 0.9534 85.54 2.90 |
| Makoid-Banakar | kMB n c R2adj. AIC MSC | 4.836 0.220 −0.006 0.9912 60.45 4.44 | 2.905 0.310 −0.002 0.9976 33.88 5.77 |
| Kinetic Model | Parameter | PLA-BCA-2% | PLA-BCA-5% |
|---|---|---|---|
| Zero order with F0 | k0 F0 R2adj. AIC MSC | 0.195 5.814 0.988 45.66 4.22 | 0.097 4.694 0.997 21.61 5.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajić, I.; Stojanović, S.; Ristić, I.; Ilić-Stojanović, S.; Pilić, B.; Nešić, A.; Najman, S.; Dinić, A.; Stanojević, L.; Urošević, M.; et al. Electrospun Poly(lactide) Fibers as Carriers for Controlled Release of Biochanin A. Pharmaceutics 2022, 14, 528. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14030528
Gajić I, Stojanović S, Ristić I, Ilić-Stojanović S, Pilić B, Nešić A, Najman S, Dinić A, Stanojević L, Urošević M, et al. Electrospun Poly(lactide) Fibers as Carriers for Controlled Release of Biochanin A. Pharmaceutics. 2022; 14(3):528. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14030528
Chicago/Turabian StyleGajić, Ivana, Sanja Stojanović, Ivan Ristić, Snežana Ilić-Stojanović, Branka Pilić, Aleksandra Nešić, Stevo Najman, Ana Dinić, Ljiljana Stanojević, Maja Urošević, and et al. 2022. "Electrospun Poly(lactide) Fibers as Carriers for Controlled Release of Biochanin A" Pharmaceutics 14, no. 3: 528. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics14030528