Cadmium Uptake by Wheat (Triticum aestivum L.): An Overview
Abstract
:1. Introduction
2. Cadmium Transport in Wheat
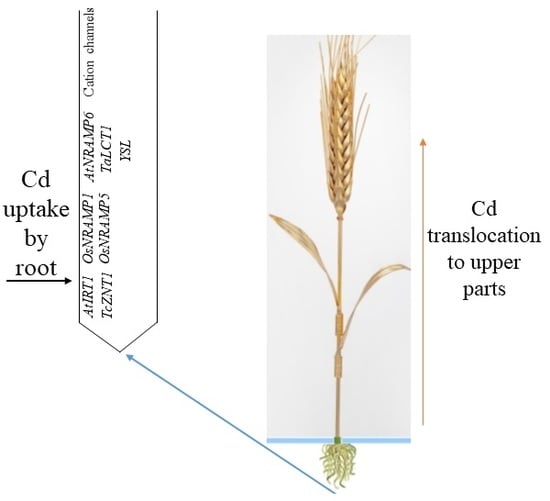
2.1. Cd Entry into the Roots
2.1.1. Transporters Involved in Cd Entry into the Roots
2.1.2. Cation Channels Involved in Cd Entry into the Roots and Cd Chelate Entry into the Roots via YSL
2.2. Cadmium entry into Shoots and Grains
3. Cd Phytotoxicity and Detoxification Mechanism in Wheat
4. Effects of Different Parameters on Reducing Cd Uptake by Plants
5. Agronomic Techniques for Decreasing the Uptake and Accumulation of Cd by Plants
5.1. Sulfur-Based Fertilizers
5.2. Using Si
5.3. Using Zinc
5.4. Using Organic Amendment
5.5. Using Bacteria
6. Conclusions
- Cadmium enters the root of wheat via transporters (NRAMP, ZIP, and low-affinity calcium transporters), cation channels (DACCs, HACCs, and VICCs), and Cd chelates via YSL;
- Cadmium may easily reach plants via root uptake and translocation to shoots and grains because of its high mobility;
- Several agronomic techniques can be used to reduce Cd uptake by wheat, the most effective of which is the use of biochar, compared to other techniques, such as using bacteria or silicon.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Konate, A.; He, X.; Zhang, Z.; Ma, Y.; Zhang, P.; Alugongo, G.M.; Rui, Y. Magnetic (Fe3O4) Nanoparticles Reduce Heavy Metals Uptake and Mitigate Their Toxicity in Wheat Seedling. Sustainability 2017, 9, 790. [Google Scholar] [CrossRef] [Green Version]
- Dong, Q.; Fang, J.; Huang, F.; Cai, K. Silicon Amendment Reduces Soil Cd Availability and Cd Uptake of Two Pennisetum Species. Int. J. Environ. Res. Public Health 2019, 16, 1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, M.Z.U.; Zafar, M.; Waris, A.A.; Rizwan, M.; Ali, S.; Sabir, M.; Usman, M.; Ayub, M.A.; Ahmad, Z. Residual effects of frequently available organic amendments on cadmium bioavailability and accumulation in wheat. Chemosphere 2020, 244, 125548. [Google Scholar] [CrossRef] [PubMed]
- Page, V.; Feller, U. Heavy Metals in Crop Plants: Transport and Redistribution Processes on the Whole Plant Level. Agronomy 2015, 5, 447–463. [Google Scholar] [CrossRef] [Green Version]
- Shafiq, S.; Zeb, Q.; Ali, A.; Sajjad, Y.; Nazir, R.; Widemann, E.; Liu, L. Lead, Cadmium and Zinc Phytotoxicity Alter DNA Methylation Levels to Confer Heavy Metal Tolerance in Wheat. Int. J. Mol. Sci. 2019, 20, 4676. [Google Scholar] [CrossRef] [Green Version]
- Retamal-Salgado, J.; Hirzel, J.; Walter, I.; Matus, I. Bioabsorption and Bioaccumulation of Cadmium in the Straw and Grain of Maize (Zea mays L.) in Growing Soils Contaminated with Cadmium in Different Environment. Int. J. Environ. Res. Public Health 2017, 14, 1399. [Google Scholar] [CrossRef] [Green Version]
- Guo, G.; Lei, M.; Wang, Y.; Song, B.; Yang, J. Accumulation of As, Cd, and Pb in Sixteen Wheat Cultivars Grown in Contaminated Soils and Associated Health Risk Assessment. Int. J. Environ. Res. Public Health 2018, 15, 2601. [Google Scholar] [CrossRef] [Green Version]
- Sobolewska, M.; Wenda-Piesik, A.; Jaroszewska, A.; Stankowski, S. Effect of Habitat and Foliar Fertilization with K, Zn and Mn on Winter Wheat Grain and Baking Qualities. Agronomy 2020, 10, 276. [Google Scholar] [CrossRef] [Green Version]
- Vitale, J.; Adam, B.; Vitale, P. Economics of Wheat Breeding Strategies: Focusing on Oklahoma Hard Red Winter Wheat. Agronomy 2020, 10, 238. [Google Scholar] [CrossRef] [Green Version]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-Ur-Rehman, M.; Qayyum, M.F.; Abbas, F.; Hannan, F.; Rinklebe, J.; Ok, Y.S. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef]
- Chunhabundit, R. Cadmium Exposure and Potential Health Risk from Foods in Contaminated Area, Thailand. Toxicol. Res. 2016, 32, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Cao, X.; Pan, J.; Li, T.; Khan, M.B.; Gurajala, H.K.; He, Z.; Yang, X. Identification of wheat (Triticum aestivum L.) genotypes for food safety on two different cadmium contaminated soils. Environ. Sci. Pollut. Res. 2020, 27, 7943–7956. [Google Scholar] [CrossRef]
- Huang, M.; Zhou, S.; Sun, B.; Zhao, Q. Heavy metals in wheat grain: Assessment of potential health risk for inhabitants in Kunshan, China. Sci. Total Environ. 2008, 405, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Grant, C.A. Cadmium and Zinc Concentration in Grain of Durum Wheat in Relation to Phosphorus Fertilization, Crop Sequence and Tillage Management. Appl. Environ. Soil Sci. 2012, 2012, 817107. [Google Scholar] [CrossRef] [Green Version]
- Corguinha, A.P.B.; de Souza, G.A.; Gonçalves, V.C.; Carvalho, C.D.A.; de Lima, W.E.A.; Martins, F.A.D.; Yamanaka, C.H.; Francisco, E.A.B.; Guilherme, L.R.G. Assessing arsenic, cadmium, and lead contents in major crops in Brazil for food safety purposes. J. Food Compos. Anal. 2015, 37, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Payandeh, K.; Jafarnejadi, A.; Gholami, A.; Shokohfar, A.; Panahpor, E. Evaluation of Cadmium Concentration in Wheat Crop Affected by Cropping System. Jundishahpur J. Health Sci. 2018, 10, e14400. [Google Scholar] [CrossRef] [Green Version]
- Khoshgoftarmanesh, A.H.; Shariatmadari, H.; Karimian, N.; Kalbasi, M.; van der Zee, S.E.A.T.M. Cadmium and Zinc in Saline Soil Solutions and their Concentrations in Wheat. Soil Sci. Soc. Am. J. 2006, 70, 582. [Google Scholar] [CrossRef]
- Awan, N.; Fatima, A.; Farhan, M.; Awan, N. Comparative Analysis of Chromium and Cadmium in Various Parts of Wheat and Maize. Pol. J. Environ. Stud. 2019, 28, 1561–1566. [Google Scholar] [CrossRef]
- Oliver, D.P.; Gore, P.J.; Moss, H.J.; Tiller, K.G. Cadmium in Wheat-Grain and Milling Products from some Australian Flour Mills. Aust. J. Agric. Res. 1993, 44, 1–11. [Google Scholar] [CrossRef]
- Lux, A.; Martinka, M.; Vaculík, M.; White, P.J. Root responses to cadmium in the rhizosphere: A review. J. Exp. Bot. 2011, 62, 21–37. [Google Scholar] [CrossRef] [Green Version]
- Begum, N.; Hu, Z.; Cai, Q.; Lou, L. Influence of PGPB Inoculation on HSP70 and HMA3 Gene Expression in Switchgrass under Cadmium Stress. Plants 2019, 8, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Jin, L.; Wang, X. Cadmium absorption and transportation pathways in plants. Int. J. Phytoremediat. 2017, 19, 133–141. [Google Scholar] [CrossRef]
- Huang, X.; Duan, S.; Wu, Q.; Yu, M.; Shabala, S. Reducing Cadmium Accumulation in Plants: Structure–Function Relations and Tissue-Specific Operation of Transporters in the Spotlight. Plants 2020, 9, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, S.; Tsuzuki, C.; Kato, A.; Aisu, A.; Yoshida, J.; Mizuno, T. AtIRT1, the Primary Iron Uptake Transporter in the Root, Mediates Excess Nickel Accumulation in Arabidopsis thaliana. Plant Cell Physiol. 2011, 52, 11433–11442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaza, S.; Tearall, K.L.; Zhao, F.J.; Buchner, P.; McGrath, S.P.; Hawkesford, M.J. Expression and functional analysis of metal transporter genes in two contrasting ecotypes of the hyperaccumulator Thlaspi caerulescens. J. Exp. Bot. 2007, 58, 1717–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Tomar, R.S.; Grag, D.; Rao, V.P.; Sharma, M.K.; Sengar, R.S. phylogenetic analysis (in-silico) of natural resistance-associated macrophage protein (NRAMP) and identification of its homoLog in bread wheat (Triticum aestivum L.). Int. J. Appl. Biol. Pharm. Technol. 2016, 7, 228–238. [Google Scholar]
- Uraguchi, S.; Fujiwara, T. Cadmium transport and tolerance in rice: Perspectives for reducing grain cadmium accumulation. Rice 2012, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Peng, F.; Wang, C.; Cheng, Y.; Kang, H.; Fan, X.; Sha, L.; Zhang, H.; Zeng, J.; Zhou, Y.; Wang, Y. Cloning and Characterization of TpNRAMP3, a Metal Transporter from Polish Wheat (Triticum polonicum L.). Front. Plant Sci. 2018, 9, 1354. [Google Scholar] [CrossRef]
- Clemens, S.; Antosiewicz, D.M.; Ward, J.M.; Schachtman, D.P.; Schroeder, J.I. The plant cDNA LCT1 mediates the uptake of calcium and cadmium in yeast. Proc. Natl. Acad. Sci. USA 1998, 95, 12043–12048. [Google Scholar] [CrossRef] [Green Version]
- Uraguchi, S.; Kamiya, T.; Sakamoto, T.; Kasai, K.; Sato, Y.; Nagamura, Y.; Yoshida, A.; Kyozuka, J.; Ishikawa, S.; Fujiwara, T. Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc. Natl. Acad. Sci. USA 2011, 108, 20959–20964. [Google Scholar] [CrossRef] [Green Version]
- Jammes, F.; Hu, H.C.; Villiers, F.; Bouten, R.; Kwak, J.M. Calcium-permeable channels in plant cells. FEBS J. 2011, 278, 4262–6276. [Google Scholar] [CrossRef] [PubMed]
- Vriese, K.D.; Costa, A.; Beeckman, T.; Vanneste, S. Pharmacological Strategies for Manipulating Plant Ca2+ Signalling. Int. J. Mol. Sci. 2018, 19, 1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miedema, H.; Demidchik, V.; Véry, A.A.; Bothwell, J.H.F.; Brownlee, C.; Davies, J.M. Two voltage-dependent calcium channels co-exist in the apical plasma membrane of Arabidopsis thaliana root hairs. New Phytologist 2008, 179, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ouyang, Y.; Fan, Y.; Qiu, B.; Zhang, G.; Zeng, F. The pathway of transmembrane cadmium influx via calcium-permeable channels and its spatial characteristics along rice root. J. Exp. Bot. 2018, 69, 5279–5291. [Google Scholar] [CrossRef] [Green Version]
- Curie, C.; Cassin, G.; Couch, D.; Divol, F.; Higuchi, K.; Jean, M.L.; Misson, J.; Schikora, A.; Czernic, P.; Mari, S. Metal movement within the plant: Contribution of nicotianamine and yellow stripe 1-like transporters. Ann. Bot. 2009, 103, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Araki, R.; Murata, J.; Murata, Y. A Novel Barley Yellow Stripe 1-Like Transporter (HvYSL2) Localized to the Root Endodermis Transports Metal–Phytosiderophore Complexes. Plant Cell Physiol. 2011, 52, 1931–1940. [Google Scholar] [CrossRef] [Green Version]
- Kuriakose, S.V.; Prasad, M.N.V. Cadmium as an environmental contaminant: Consequences to plant and human health. In Trace Elements as Contaminants and Nutrients; Prasad, M.V.N., Ed.; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Jiao, Y.; Grant, C.A.; Bailey, L. Effects of phosphorus and zinc fertilizer on cadmium uptake and distribution in flax and durum wheat. J. Sci. Food Agric. 2004, 84, 777–785. [Google Scholar] [CrossRef]
- Qiao, K.; Wang, F.; Liang, S.; Wang, H.; Hu, Z.; Chai, T. Improved Cd, Zn and Mn tolerance and reduced Cd accumulation in grains with wheat-based cell number regulator TaCNR2. Sci. Rep. 2019, 9, 870. [Google Scholar] [CrossRef] [Green Version]
- Padilla-Benavides, T.; McCann, C.J.; Argüello, J.M. The Mechanism of Cu+ Transport ATPases. J. Bio. Chem. 2013, 288, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Parameswaran, A.; Leitenmaier, B.; Yang, M.; Kroneck, P.M.; Welte, W.; Lutz, G.; Papoyan, A.; Kochian, L.V.; Küpper, H. A native Zn/Cd pumping P1B ATPase from natural overexpression in a hyperaccumulator plant. Biochem. Biophys. Res. Commun. 2007, 363, 51–56. [Google Scholar] [CrossRef]
- Argüello, J.M.; Gonzalez-Guerrero, M.; Raimunda, D. Bacterial Transition Metal P1B-ATPases: Transport Mechanism and Roles in Virulence. Biochemistry 2011, 50, 9940–9949. [Google Scholar] [CrossRef] [Green Version]
- Hart, J.J.; Welch, R.M.; Norvell, W.A.; Sullivan, L.A.; Kochian, L.V. Characterization of Cadmium Binding, Uptake, and Translocation in Intact Seedlings of Bread and Durum Wheat Cultivars. Plant Physiol. 1998, 116, 1413–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Islam, M.A.; Lin, H.; Ji, C.; Duan, Y.; Liu, P.; Zeng, Q.; Day, B.; Kang, Z.; Guo, J. Genome-Wide Identification of Cyclic Nucleotide-Gated Ion Channel Gene Family in Wheat and Functional Analyses of TaCNGC14 and TaCNGC16. Front. Plant Sci. 2018, 9, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.Y.; Belloeil, C.; Ianna, M.L.; Shin, R. Arabidopsis CNGC Family Members Contribute to Heavy Metal Ion Uptake in Plants. Int. J. Mol. Sci. 2019, 20, 413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Zhou, Q.; Cai, Z. Effect of soil HHCB on cadmium accumulation and phytotoxicity in wheat seedlings. Ecotoxicology 2014, 23, 1996–2004. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, O.; Abbaspour, H. Effects of methyl jasmonate and cadmium on growth traits, cadmium transport and accumulation, and allene-oxide cyclase gene expression in wheat seedlings. Revista Agric. Neotrop. Cassilândia MS 2019, 6, 20–29. [Google Scholar] [CrossRef] [Green Version]
- He, L.; Wang, X.; Feng, R.; He, Q.; Wang, S.; Liang, C.; Yan, L.; Bi, Y. Alternative Pathway is Involved in Nitric Oxide-Enhanced Tolerance to Cadmium Stress in Barley Roots. Plants 2019, 8, 557. [Google Scholar] [CrossRef] [Green Version]
- DalCorso, G.; Farinati, S.; Maistri, S.; Furini, A. How Plants Cope with Cadmium: Staking All on Metabolism and Gene Expression. J. Integr. Plant Biol. 2008, 50, 1268–1280. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. MoLecular Mechanism of Heavy Metal Toxicity and Tolerance in Plants: Central Role of Glutathione in Detoxification of Reactive Oxygen Species and Methylglyoxal and in Heavy Metal Chelation. J. Bot. 2012, 2012, 872875. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Rehman, M.Z.U.; Farid, M.; Asma, M. Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef]
- Azevedo, R.A.; Gratão, P.L.; Monteiro, C.C.; Carvalho, R.F. What is new in the research on cadmium-induced stress in plants? Food Energy Secur. 2012, 1, 133–140. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Yang, L.; Li, H. Dynamics of rhizosphere properties and antioxidative responses in wheat (Triticum aestivum L.) under cadmium stress. Ecotoxicol. Environ. Saf. 2014, 102, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Aprile, A.; Sabella, E.; Francia, E.; Milc, J.; Ronga, D.; Pecchioni, N.; Ferrari, E.; Luvisi, A.; Vergine, M.; Bellis, L.D. Combined Effect of Cadmium and Lead on Durum Wheat. Int. J. Mol. Sci. 2019, 20, 5891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aprile, A.; Sabella, E.; Vergine, M.; Genga, A.; Siciliano, M.; Nutricati, E.; Rampino, P.; De Pascali, M.; Luvisi, A.; Miceli, A.; et al. Activation of a gene network in durum wheat roots exposed to cadmium. BMC Plant Biol. 2018, 18, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiDonato, R.J., Jr.; Roberts, L.A.; Sanderson, T.; Eisley, R.B.; Walker, E.L. Arabidopsis Yellow Stripe-Like2 (YSL2): A metal-regulated gene encoding a plasma membrane transporter of nicotianamine–metal complexes. Plant J. 2004, 39, 403–414. [Google Scholar] [CrossRef]
- Stolt, P.; Asp, H.; Hultin, S. Genetic variation in wheat cadmium accumulation on soils with different cadmium concentrations. J. Agron. Crop. Sci. 2006, 192, 201–208. [Google Scholar] [CrossRef]
- Liu, K.; Lv, J.; He, W.; Zhang, H.; Cao, Y.; Dai, Y. Major factors influencing cadmium uptake from the soil into wheat plants. Ecotoxicol. Environ. Saf. 2015, 113, 207–2013. [Google Scholar] [CrossRef]
- Sebastian, A.; Prasad, M.N.V. Cadmium minimization in rice. A review. Agron. Sustain. Dev. 2014, 34, 155–173. [Google Scholar] [CrossRef]
- Nylund, E. Cadmium Uptake in Willow (Salix viminalis L.) and Spring Wheat (Triticum aestivum L.) in Relation to Plant Growth and Cd Concentration in Soil Solution. Ph.D. Thesis, Department of Soil Science, Swedish University of Agricultural Sciences, Swedish, Sweden, 2003. [Google Scholar]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef]
- Ghaley, B.B.; Wösten, H.; Olesen, J.E.; Schelde, K.; Baby, S.; Karki, Y.K.; Børgesen, C.D.; Smith, P.; Yeluripati, J.; Ferrise, R.; et al. Simulation of Soil Organic Carbon Effects on Long-Term Winter Wheat (Triticum aestivum) Production Under Varying Fertilizer Inputs. Front. Plant Sci. 2019, 9, 1158. [Google Scholar] [CrossRef] [Green Version]
- Abedi, T.; Mojiri, A. Arsenic Uptake and Accumulation Mechanisms in Rice Species. Plants 2020, 9, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hucker, C.A. Organic Amendments for Reducing the Plant Uptake of Cadmium from New Zealand Soils. Ph.D. Thesis, Lincoln University, Lincoln, New Zealand, 2016. [Google Scholar]
- Hassan, M.J.; Wang, F.; Ali, S.; Zhang, G. Toxic Effect of Cadmium on Rice as Affected by Nitrogen Fertilizer Form. Plant Soil 2005, 277, 359–365. [Google Scholar] [CrossRef]
- Konotop, Y.; Mezsaros, P.; Matušíková, I.; Batsmanova, L.; Taran, N. Application of nitrogen nutrition for improving tolerance of soybean seedlings to cadmium. Environ. Exp. Biol. 2012, 10, 139–144. [Google Scholar]
- Mitchell, L.G.; Grant, C.A.; Racz, G.J. Effect of nitrogen application on concentration of cadmium and nutrient ions in soil solution and in durum wheat. Can. J. Soil Sci. 2000, 80, 107–115. [Google Scholar] [CrossRef]
- Wångstrand, H.; Eriksson, J.; Öborn, I. Cadmium concentration in winter wheat as affected by nitrogen fertilization. Eur. J. Agron. 2007, 26, 209–214. [Google Scholar] [CrossRef]
- Li, X.; Ziadi, N.; Bélanger, G.; Yuan, W.; Liang, S.; Xu, H.; Cai, Z. Wheat grain Cd concentration and uptake as affected by timing of fertilizer N application. Can. J. Soil Sci. 2013, 93, 219–222. [Google Scholar] [CrossRef]
- Landberg, T.; Greger, M. Influence of N and N supplementation on Cd accumulation in wheat grain. In Proceedings of the 7th International Conference on the Biogeochemistry of Trace Elements, Uppsala ’03, Conference Proceedings, Uppsala, Sweden, 15–19 June 2003; pp. 90–91. [Google Scholar]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, M.; Mahmood, A.; Zia-Ur-Rehman, M.; Ibrahim, M.; Arshad, M.; Qayyum, M.F. Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol. Environ. Saf. 2018, 148, 825–833. [Google Scholar] [CrossRef]
- Sheng, H.; Zeng, J.; Liu, Y.; Wang, X.; Wang, Y.; Kang, H.; Fan, X.; Sha, L.; Zhang, H.; Zhou, Y. Sulfur Mediated Alleviation of Mn Toxicity in Polish Wheat Relates to Regulating Mn Allocation and Improving Antioxidant System. Front. Plant Sci. 2016, 7, 1382. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.; Lu, H.; Liu, H.; Lou, L.; Zhang, P.; Song, G.; Zhou, H.; Ma, H. Sulfate application decreases translocation of arsenic and cadmium within wheat (Triticum aestivum L.) plant. Sci. Total Environ. 2020, 713, 136665. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Q.F.; Li, J.; Xiong, J.; Zhou, L.; He, S.L.; Zhang, J.Q.; Chen, Z.; He, S.G.; Liu, H. Effects of exogenous sulfur on alleviating cadmium stress in tartary buckwheat. Sci. Rep. 2019, 9, 7397. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Wen, X.H.; Cai, Y.X.; Cai, K.Z. Silicon-Mediated Enhancement of Heavy Metal Tolerance in Rice at Different Growth Stages. Int. J. Environ. Res. Public Health 2018, 15, 2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, I.; Ashraf, M.A.; Rasheed, R.; Asghar, A.; Sajid, M.A.; Iqbal, M. Exogenous application of silicon at the boot stage decreases accumulation of cadmium in wheat (Triticum aestivum L.) grains. Braz. J. Bot. 2015, 38, 223–234. [Google Scholar] [CrossRef]
- Bhat, J.A.; Shivaraj, S.M.; Singh, P.; Navadagi, D.B.; Tripathi, D.K.; Dash, P.K.; Solanke, A.U.; Sonah, H.; Deshmukh, R. Role of Silicon in Mitigation of Heavy Metal Stresses in Crop Plants. Plants 2019, 8, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naeem, A.; Saifullah, A.; Zia-ur-Rehman, M.; Aktar, T.; Zia, M.H.; Aslam, M. Silicon nutrition lowers cadmium content of wheat cultivars by regulating transpiration rate and activity of antioxidant enzymes. Environ. Pollut. 2018, 242, 126–135. [Google Scholar] [CrossRef]
- Saifullah, A.; Javed, H.; Naeem, A.; Rengel, Z.; Dahlawi, S. Timing of foliar Zn application plays a vital role in minimizing Cd accumulation in wheat. Environ. Sci. Pollut. Res. Int. 2016, 23, 16432–16439. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Zhu, Y.G.; Smith, F.A.; Smith, S.E. Cadmium Uptake by Winter Wheat Seedlings in Response to Interactions Between Phosphorus and Zinc Supply in Soils. J. Plant Nutr. 2005, 28, 1569–1580. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, B.; Liu, H.; Liang, X.; Ma, W.; Shi, Z.; Yang, S. Zinc effects on cadmium toxicity in two wheat varieties (Triticum aestivum L.) differing in grain cadmium accumulation. Ecotoxicol. Environ. Saf. 2019, 183, 109562. [Google Scholar] [CrossRef]
- Bashir, A.; Rizwan, M.; Rehman, M.Z.U.; Zubair, M.; Riaz, M.; Qayyum, M.F.; Alharby, H.F.; Bamagoos, A.A.; Ali, S. Application of co-composted farm manure and biochar increased the wheat growth and decreased cadmium accumulation in plants under different water regimes. Chemosphere 2020, 246, 125809. [Google Scholar] [CrossRef]
- Lwin, C.S.; Seo, B.H.; Kim, H.U.; Owens, G.; Kim, K.R. Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality—A critical review. Soil Sci. Plant Nutr. 2018, 64, 156–167. [Google Scholar] [CrossRef]
- Li, M.; Mohamed, I.; Raleve, D.; Chen, W.; Huang, Q. Field evaluation of intensive compost application on Cd fractionation and phytoavailability in a mining-contaminated soil. Environ. Geochem. Health 2016, 38, 1193–1201. [Google Scholar] [CrossRef]
- Khedr, M.E.; Nasseem, M.G.; Ali, W.H.; Rashad, M.A. Compost and Vermicompost as Soil Amendments to Immobilize Cu and Cd Under Wheat Growth Conditions. Alex. Sci. Exch. J. 2019, 40, 705–716. [Google Scholar] [CrossRef]
- Sato, A.; Takeda, H.; Oyanagi, W.; Nishihara, E.; Murakami, M. Reduction of cadmium uptake in spinach (Spinacia oleracea L.) by soil amendment with animal waste compost. J. Hazard. Mater. 2010, 181, 298–304. [Google Scholar] [CrossRef]
- Rehman, M.Z.U.; Rizwan, M.; Hussain, A.; Saqib, M.; Ali, S.; Sohail, M.I.; Shafiq, M.; Hafeez, F. Alleviation of cadmium (Cd) toxicity and minimizing its uptake in wheat (Triticum aestivum) by using organic carbon sources in Cd-spiked soil. Environ. Pollut. 2018, 241, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Erdem, H.; Kinay, A.; Günal, E.; Yaban, H.; Tutus, Y. The effects of biochar application on cadmium uptake of tobacco. Carpath. J. Earth Environ. 2011, 12, 447–456. [Google Scholar]
- Sun, J.; Fan, Q.; Ma, J.; Cui, L.; Quan, G.; Yan, J. Wu, L.; Hina, K.; Abdul, B.; Wang, H. Effects of biochar on cadmium (Cd) uptake in vegetables and its natural downward movement in saline-alkali soil. Environ. Pollut. Bioavailab. 2020, 32, 36–46. [Google Scholar] [CrossRef]
- Wang, X.H.; Wang, Q.; Nie, Z.W.; He, L.H.; Sheng, X.F. Ralstonia eutropha Q2-8 reduces wheat plant above-ground tissue cadmium and arsenic uptake and increases the expression of the plant root cell wall organization and biosynthesis-related proteins. Environ. Pollut. 2018, 242, 1488–1499. [Google Scholar] [CrossRef]
- Mallick, I.; Bhattacharyya, C.; Mukherji, S.; Dey, D.; Sarkar, S.C.; Mukhopadhyay, U.K.; Ghosh, A. Effective rhizoinoculation and biofilm formation by arsenic immobilizing halophilic plant growth promoting bacteria (PGPB) isolated from mangrove rhizosphere: A step towards arsenic rhizoremediation. Sci. Total Environ. 2018, 610, 1239–1250. [Google Scholar] [CrossRef]
- Wang, X.H.; Luo, W.W.; Wang, Q.; He, L.Y.; Sheng, F. Metal(loid)-resistant bacteria reduce wheat Cd and As uptake in metal(loid)-contaminated soil. Environ. Pollut. 2018, 241, 529–539. [Google Scholar] [CrossRef]
- Wang, X.; Nie, Z.; He, L.; Wang, Q.; Sheng, X. Isolation of As-tolerant bacteria and their potentials of reducing As and Cd accumulation of edible tissues of vegetables in metal(loid)-contaminated soils. Sci. Total Environ. 2017, 579, 179–189. [Google Scholar] [CrossRef]
- Ahemad, M. Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: Paradigms and prospects. Arab. J. Chem. 2019, 12, 1365–1377. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Mou, R.; Cao, Z.; Xu, P.; Wu, X.; Zhu, Z.; Chen, M. Characterization of cadmium-resistant bacteria and their potential for reducing accumulation of cadmium in rice grains. Sci. Total Environ. 2016, 569–570, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Khan, M.A.; Asaf, S.; Lubna, N.; Lee, I.J.; Kim, K.M. Metal Resistant Endophytic Bacteria Reduces Cadmium, Nickel Toxicity, and Enhances Expression of Metal Stress Related Genes with Improved Growth of Oryza Sativa, via Regulating Its Antioxidant Machinery and Endogenous Hormones. Plant 2019, 8, 363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohail, M.I.; Rehman, M.Z.U.; Rizwan, M.; Yousaf, B.; Ali, S.; Haq, M.A.U.; Anayat, A.; Waris, A.A. Efficiency of various silicon rich amendments on growth and cadmium accumulation in field grown cereals and health risk assessment. Chemosphere 2020, 244, 125481. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.Z.U.; Khalid, H.; Akmal, F.; Ali, S.; Rizwan, M.; Qayyum, M.F.; Iqbal, M.; Khalid, M.U.; Azhar, M. Effect of limestone, lignite and biochar applied alone and combined on cadmium uptake in wheat and rice under rotation in an effluent irrigated field. Environ. Pollut. 2017, 227, 560–568. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; Rehman, M.Z.U.; Javed, M.R.; Imran, M.; Chatha, S.A.S.; Nazir, R. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ. Pollut. 2018, 242, 1518–1526. [Google Scholar] [CrossRef]
- Wu, C.; Dun, Y.; Zhang, Z.; Li, M.; Wu, G. Foliar application of selenium and zinc to alleviate wheat (Triticum aestivum L.) cadmium toxicity and uptake from cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 190, 110091. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Hussain, A.; Rehman, M.Z.U.; Ali, B.; Yousaf, B.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2019, 140, 1–8. [Google Scholar] [CrossRef]
- Huang, H.; Rizwan, M.; Li, M.; Song, F.; Zhou, S.; He, X.; Ding, R.; Dai, Z.; Yuan, Y.; Cao, M.; et al. Comparative efficacy of organic and inorganic silicon fertilizers on antioxidant response, Cd/Pb accumulation and health risk assessment in wheat (Triticum aestivum L.). Environ. Pollut. 2019, 255, 113146. [Google Scholar] [CrossRef]
| Cd (mg/Kg) in Wheat; Average or Range | Cd (mg/Kg) in Soil; Average or Range | Soil Characteristics | Remarks | Area | Reference |
|---|---|---|---|---|---|
| 0.14 (grain) | 0.38 | pH = 5.9 CEC (cmol/Kg) = 21.3 OM (%) = NR ** Clay (%) = 15.8 | Yangmai16 * | The north of Zhejiang Province, China | [12] |
| 0.12 (grain) | 0.36 | pH = 4.9 CEC (cmol/Kg) = 34.6 OM (%) = NR Clay (%) = 117.5 | Yangmai16 | The east of Zhejiang Province, China | [12] |
| 3.17 (root) 1.11 (stem) 0.25 (grain) | 2.06 | pH = 7.5 CEC (cmol/Kg) = 7.6 OM (%) = NR Clay (%) = NR | Zhengmai7698 | Henan Province, China | [7] |
| 0.006 to 0.17 (grain) | 0.09 to 1.0 | pH = 6.6 CEC (cmol/Kg) = 18.2 OM (%) = 3.0 Clay (%) = NR | NR | Kunshan, China | [13] |
| 0.247 (grain) | 0.10 | pH = 7.5 CEC (cmol/Kg) = NR OM (%) = NR Clay (%) = NR | - | Brandon, Manitoba, Canada | [14] |
| 0.01 to 0.08 (grain) | 0.21 | pH = 5.3 CEC (cmol/Kg) = 31 OM = NR Clay (%) = NR | - | São Gotardo (MG), Brazil | [15] |
| 0.95 (root) 0.60 (stem) | 0.27 | pH = 7.8 CEC (cmol/Kg) = NR OM (%) = 0.7 Clay (%) = NR | - | Khuzestan Province, Iran | [16] |
| 0.01 to 0.02 (grain) 0.01 to 0.03 (grain) | 3.2 | pH = 7.6 CEC (cmol/Kg) = NR OM = 0.14 Clay (%) = 46 | Rushan Falat | Qom, Iran | [17] |
| 0.93 (grain) 0.16 (stem) 0.67 (root) | NR | pH = NR CEC (cmol/Kg) = NR OM = NR Clay (%) = NR | - | Lahore, Pakistan | [18] |
| 0.003 to 0.03 (grain) | NR | pH = NR CEC (cmol/Kg) = NR OM = NR Clay (%) = NR | - | Sydney, Australia | [19] |
| Name | Remarks | Reference |
|---|---|---|
| AtIRT1 | A plasma membrane transporter. Involved in entrance of Cd into root. | [24] |
| TcZNT1 | Involved in entrance of Cd to root. | [25] |
| OsNRAMP1 | Cd-influx transporter in the plasma membrane. Involved in entrance of Cd into root. | [27] |
| OsNRAMP5 | Cd-influx transporter in the plasma membrane. Involved in entrance of Cd into root. | [27] |
| AtNRAMP6 | An intracellular metal transporter. Involved in entrance of Cd into root. | [28] |
| TaLCT1 | An influx transporter in wheat. Involved in entrance of Cd into root. | [30] |
| YSL | A kind of oligopeptide transporter. Involved in entrance of Cd into root over Cd-chelates across plant cell membranes. | [35] |
| P1B-ATPases | A group of ubiquitous membranes. Transporting Cd from root to shoot. | [39] |
| CNGC gene family | Ca2+ channels in root protoplast plasma membrane. Indirectly involved in entrance of Cd into root. Responsible for coding of HACCs, VICCs, and DACCs *. | [44,45] |
| DACCs | Ca2+ channels. Involved in entrance of Cd into root. | [32] |
| HACCs | Ca2+ channels. Involved in entrance of Cd into root. | [32] |
| VICCs | Ca2+ channels. Involved in entrance of Cd into root. | [34] |
| Decreasing of Cd Accumulation in Root/Stem or Straw/Grains | Cd Concentration in Wheat after Treating (mg/Kg) | Method | Remarks | Reference |
|---|---|---|---|---|
| 48.3% (in straw) 97.8% (in grain) | 0.80 (in shoot) 0.01 (in grain) | Using rice husk biochar | Mixing silicon-rich biochar with soil | [97] |
| 54% (in root) 50% (in shoot) 65% (in grains) | 2.0 (in root) 1.1 (in shoot) 0.2 (in grain) | Using co-composted farm manure and biochar | Mixing organic amendments with soil | [82] |
| 69% (in root) 67% (in shoot) 62.5% (in grains) | 12 (in root) 2.7 (in shoot) 0.15 (in grain) | Using rice husk biochar | Mixing biochar with soil | [87] |
| 55% (in root) 51% (in shoot) | 1.2 (in root) 0.7 (in shoot) | Using biochar | Mixing biochar with soil under stress conditions | [71] |
| 57% (in grains) | 0.2 (in grain) | Using biochar | Mixing biochar (5%) with soil | [10] |
| 97% (in straw) | >0.2 (in straw) | Using limestone + biochar | Mixing limestone + biochar with soil | [98] |
| 77% (in grains) | 1.1–0.2 (in grain) | Using zinc oxide nanoparticles | Foliar application | [99] |
| 55% to 69% (in root) | 1–0.6 (in root) | Using zinc | Using ZnSO4 in nutrient solution | [81] |
| 7%–24% (in root) 13%–37% (in stem) 13%–50% (in grains) | 4–3 (in root) 3.8–2.2 (in stem) 0.2–0.9 (in grain) | Using zinc | Foliar application | [100] |
| 10%–31% (in root) 27%–52% (in shoot) 33%–70% (in grains) | 2.7–2.0 (in root) 1.6–0.9 (in shoot) 0.5–0.2 (in grain) | Using zinc–lysine | Foliar application | [51] |
| 19%–64% (in root) 11%–53% (in shoot) 20%–82% (in grains) | 12–5 (in root) 6–2 (in shoot) 1.1–0.3 (in grains) | Using silicon nanoparticles | Foliar application | [101] |
| 30% (in shoot) | 1.2 (in shoot) | Using inorganic silicon fertilizer | Mixing the fertilizer with soil | [102] |
| 24% (in grains) | 0.35 (in grain) | Using sodium sulfate | Mixing with soil | [73] |
| 40% (in root) | NR | Using bacteria | Using Ralstonia eutropha Q2-8 | [90] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedi, T.; Mojiri, A. Cadmium Uptake by Wheat (Triticum aestivum L.): An Overview. Plants 2020, 9, 500. https://0-doi-org.brum.beds.ac.uk/10.3390/plants9040500
Abedi T, Mojiri A. Cadmium Uptake by Wheat (Triticum aestivum L.): An Overview. Plants. 2020; 9(4):500. https://0-doi-org.brum.beds.ac.uk/10.3390/plants9040500
Chicago/Turabian StyleAbedi, Tayebeh, and Amin Mojiri. 2020. "Cadmium Uptake by Wheat (Triticum aestivum L.): An Overview" Plants 9, no. 4: 500. https://0-doi-org.brum.beds.ac.uk/10.3390/plants9040500