A Review on Micro- to Nanocellulose Biopolymer Scaffold Forming for Tissue Engineering Applications
Abstract
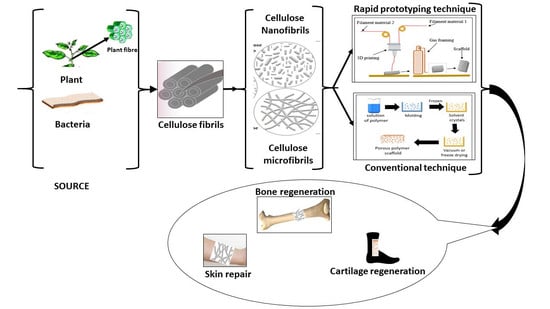
:1. Introduction
2. Plant Fibre Materials
2.1. Cellulose Fibre Architecture
2.2. Microcellulose Material Forms
2.3. Nanocellulose Material Forms
3. Cellulose-Based Scaffolds in Biomedical Engineering
3.1. Development of Cellulose-Based Scaffolding
3.2. Method of Preparation of Micro- and Nanocellulose-Based Scaffolds
3.2.1. Conventional Techniques for Micro- and Nanocellulose-Based Scaffold Fabrication
3.2.2. Rapid Prototyping Techniques for Scaffold Fabrication
3.3. Properties of Micro- and Nanocellulose-Based Blend Scaffolds
3.3.1. Physical Properties
3.3.2. Mechanical Properties
3.3.3. Biodegradability
3.3.4. Biocompatibility and Cytotoxicity
3.4. Classification of Micro/Nanocellulose-Based Scaffolds
3.4.1. Micro- and Nanocellulose-Based Binary Blends Scaffold
3.4.2. Micro- and Nanocellulose-Based Ternary Blends Scaffold
4. The New Role of Cellulose-Based Scaffolds Bioengineering
4.1. Issues and Potential
4.2. Future Prospect and Applications of Micro- and Nanocellulose-Based Scaffold
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bajwa, D.S.; Rehovsky, C.; Shojaeiarani, J.; Stark, N.; Bajwa, S.; Dietenberger, M.A. Functionalized cellulose nanocrystals: A potential fire retardant for polymer composites. Polymers 2019, 11, 1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasad, M.S.B.A.; Halim, A.S.; Hashim, K.; Rashid, A.H.A.; Yusof, N.; Shamsuddin, S. In vitro evaluation of novel chitosan derivatives sheet and paste cytocompatibility on human dermal fibroblasts. Carbohydr. Polym. 2010, 79, 1094–1100. [Google Scholar] [CrossRef]
- Abdul Khalil, H.; Adnan, A.; Yahya, E.B.; Olaiya, N.; Safrida, S.; Hossain, M.; Balakrishnan, V.; Gopakumar, D.A.; Abdullah, C.; Oyekanmi, A. A Review on Plant Cellulose Nanofibre-Based Aerogels for Biomedical Applications. Polymers 2020, 12, 1759. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.A.; Tye, Y.Y.; Leh, C.P.; Saurabh, C.; Ariffin, F.; Fizree, H.M.; Mohamed, A.; Suriani, A. Cellulose reinforced biodegradable polymer composite film for packaging applications. In Bionanocomposites for Packaging Applications; Springer: Cham, Switzerland, 2018; pp. 49–69. [Google Scholar]
- Tamaddon, F.; Hosseinzadeh, S. Anionic micro-cellulose (AMC): Preparation, characterization, and application as a novel heterogeneous base catalyst. Cellulose 2018, 25, 5277–5287. [Google Scholar] [CrossRef]
- Murphy, C.A.; Collins, M.N. Microcrystalline cellulose reinforced polylactic acid biocomposite filaments for 3D printing. Polym. Compos. 2018, 39, 1311–1320. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Auad, M.L.; Bellesi, N.E.; Nutt, S.R.; Aranguren, M.I. Cellulose micro/nanocrystals reinforced polyurethane. J. Mater. Res. 2006, 21, 870–881. [Google Scholar] [CrossRef]
- Khalil, H.A.; Tye, Y.Y.; Chow, S.T.; Saurabh, C.K.; Paridah, M.T.; Dungani, R.; Syakir, M.I. Cellulosic pulp fiber as reinforcement materials in seaweed-based film. BioResources 2017, 12, 29–42. [Google Scholar]
- Nosar, M.N.; Salehi, M.; Ghorbani, S.; Beiranvand, S.P.; Goodarzi, A.; Azami, M. Characterization of wet-electrospun cellulose acetate based 3-dimensional scaffolds for skin tissue engineering applications: Influence of cellulose acetate concentration. Cellulose 2016, 23, 3239–3248. [Google Scholar] [CrossRef]
- Sultan, S.; Mathew, A.P. 3D Printed Porous Cellulose Nanocomposite Hydrogel Scaffolds. JoVE (J. Visual. Exp.) 2019, 146, e59401. [Google Scholar] [CrossRef]
- Osorio, D.A.; Lee, B.E.; Kwiecien, J.M.; Wang, X.; Shahid, I.; Hurley, A.L.; Cranston, E.D.; Grandfield, K. Cross-linked cellulose nanocrystal aerogels as viable bone tissue scaffolds. Acta Biomater. 2019, 87, 152–165. [Google Scholar] [CrossRef]
- Naeem, M.A.; Alfred, M.; Lv, P.; Zhou, H.; Wei, Q. Three-dimensional bacterial cellulose-electrospun membrane hybrid structures fabricated through in-situ self-assembly. Cellulose 2018, 25, 6823–6830. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Athukoralalage, S.S.; Balu, R.; Dutta, N.K.; Roy Choudhury, N. 3D bioprinted nanocellulose-based hydrogels for tissue engineering applications: A brief review. Polymers 2019, 11, 898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Du, H.; Zhang, M.; Liu, K.; Liu, H.; Xie, H.; Zhang, X.; Si, C. Bacterial Cellulose-Based Composite Scaffolds for Biomedical Applications: A Review. ACS Sustain. Chem. Eng. 2020, 8, 7536–7562. [Google Scholar] [CrossRef]
- Hickey, R.J.; Pelling, A.E. Cellulose biomaterials for tissue engineering. Front. Bioeng. Biotechnol. 2019, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef]
- Rajwade, J.; Paknikar, K.; Kumbhar, J. Applications of bacterial cellulose and its composites in biomedicine. Appl. Microbiol. Biotechnol. 2015, 99, 2491–2511. [Google Scholar] [CrossRef]
- Bourmaud, A.; Beaugrand, J.; Shah, D.U.; Placet, V.; Baley, C. Towards the design of high-performance plant fibre composites. Prog. Mater. Sci. 2018, 97, 347–408. [Google Scholar] [CrossRef]
- Zhong, Y.; Kureemun, U.; Tran, L.Q.N.; Lee, H.P. Natural plant fiber composites-constituent properties and challenges in numerical modeling and simulations. Int. J. Appl. Mech. 2017, 9, 1750045. [Google Scholar] [CrossRef]
- Bourmaud, A.; Mayer-Laigle, C.; Baley, C.; Beaugrand, J. About the frontier between filling and reinforcement by fine flax particles in plant fibre composites. Ind. Crops Prod. 2019, 141, 111774. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [Green Version]
- Hurtado, P.L.; Rouilly, A.; Vandenbossche, V.; Raynaud, C. A review on the properties of cellulose fibre insulation. Build. Environ. 2016, 96, 170–177. [Google Scholar] [CrossRef] [Green Version]
- Mahltig, B.; Günther, K.; Askani, A.; Bohnet, F.; Brinkert, N.; Kyosev, Y.; Weide, T.; Krieg, M.; Leisegang, T. X-ray-protective organic/inorganic fiber–along the textile chain from fiber production to clothing application. J. Text. Inst. 2017, 108, 1975–1984. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef]
- Seabra, A.B.; Bernardes, J.S.; Fávaro, W.J.; Paula, A.J.; Durán, N. Cellulose nanocrystals as carriers in medicine and their toxicities: A review. Carbohydr. Polym. 2018, 181, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Madsen, B.; Gamstedt, E.K. Wood versus plant fibers: Similarities and differences in composite applications. Adv. Mater. Sci. Eng. 2013, 2013, 564346. [Google Scholar] [CrossRef] [Green Version]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Yang, F.; Shao, Y.; Zhang, X.; Dai, K. Modification and evaluation of micro-nano structured porous bacterial cellulose scaffold for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 1034–1041. [Google Scholar] [CrossRef]
- Pacheco, G.; de Mello, C.V.; Chiari-Andréo, B.G.; Isaac, V.L.B.; Ribeiro, S.J.L.; Pecoraro, É.; Trovatti, E. Bacterial cellulose skin masks—Properties and sensory tests. J. Cosm. Dermatol. 2018, 17, 840–847. [Google Scholar] [CrossRef]
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. J. Saudi Chem. Soc. 2018, 22, 949–978. [Google Scholar] [CrossRef]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- De Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; Ribeiro, F.d.A.S.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M. Plant and bacterial nanocellulose: Production, properties and applications in medicine, food, cosmetics, electronics and engineering. A review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Jozala, A.F.; de Lencastre-Novaes, L.C.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa, A., Jr.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072. [Google Scholar] [CrossRef] [Green Version]
- O’Connell, D.W.; Birkinshaw, C.; O’Dwyer, T.F. Heavy metal adsorbents prepared from the modification of cellulose: A review. Bioresour. Technol. 2008, 99, 6709–6724. [Google Scholar] [CrossRef]
- Borges, J.; Canejo, J.; Fernandes, S.; Brogueira, P.; Godinho, M. Cellulose-based liquid crystalline composite systems. Nanocell. Polym. Nanocompos. Fundam. Appl. Wiley Scrivener 2015, 215–235. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Chuin, C.T.H.; Sabar, S.; Fazita, M.N.; Taiwo, O.F.; Hassan, T.; Haafiz, M.M. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef]
- Sotnikova, Y.S.; Demina, T.; Istomin, A.; Goncharuk, G.; Grandfils, C.; Akopova, T.; Zelenetskii, A.; Babayevsky, P. Application of micro-and nanocrystalline cellulose. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Moscow, Russia, 21–24 November 2017. [Google Scholar]
- De Campos, A.; Corrêa, A.C.; Claro, P.I.C.; de Morais Teixeira, E.; Marconcini, J.M. Processing, Characterization and Application of Micro and Nanocellulose Based Environmentally Friendly Polymer Composites. In Sustainable Polymer Composites and Nanocomposites; Springer: Cham, Switzerland, 2019; pp. 1–35. [Google Scholar]
- Wang, T.; Hong, M. Solid-state NMR investigations of cellulose structure and interactions with matrix polysaccharides in plant primary cell walls. J. Exp. Bot. 2016, 67, 503–514. [Google Scholar] [CrossRef] [Green Version]
- Sorieul, M.; Dickson, A.; Hill, S.J.; Pearson, H. Plant fibre: Molecular structure and biomechanical properties, of a complex living material, influencing its deconstruction towards a biobased composite. Materials 2016, 9, 618. [Google Scholar] [CrossRef]
- Sreekumar, P.; Manirul Haque, S.; Afzal, H.M.; Sadique, Z.; Al-Harthi, M.A. Preparation and characterization of microcellulose reinforced polyvinyl alcohol/starch biocomposites. J. Compos. Mater. 2019, 53, 1933–1939. [Google Scholar] [CrossRef]
- Oehme, D.P.; Doblin, M.S.; Wagner, J.; Bacic, A.; Downton, M.T.; Gidley, M.J. Gaining insight into cell wall cellulose macrofibril organisation by simulating microfibril adsorption. Cellulose 2015, 22, 3501–3520. [Google Scholar] [CrossRef]
- Kataja, M.; Haavisto, S.; Salmela, J.; Lehto, R.; Koponen, A. Characterization of micro-fibrillated cellulose fiber suspension flow using multi scale velocity profile measurements. Nord. Pulp. Pap. Res. J. 2017, 32, 473–482. [Google Scholar] [CrossRef]
- Saputro, A.; Verawati, I.; Ramahdita, G.; Chalid, M. Preparation of micro-fibrillated cellulose based on sugar palm ijuk (Arenga pinnata) fibres through partial acid hydrolysis. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Medan, Indonesia, 7–10 November 2016; p. 012042. [Google Scholar]
- Nie, K.; Song, Y.; Liu, S.; Han, G.; Ben, H.; Ragauskas, A.J.; Jiang, W. Preparation and Characterization of Microcellulose and Nanocellulose Fibers from Artemisia Vulgaris Bast. Polymers 2019, 11, 907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akaraonye, E.; Filip, J.; Safarikova, M.; Salih, V.; Keshavarz, T.; Knowles, J.C.; Roy, I. Composite scaffolds for cartilage tissue engineering based on natural polymers of bacterial origin, thermoplastic poly (3--hydroxybutyrate) and micro--fibrillated bacterial cellulose. Polym. Int. 2016, 65, 780–791. [Google Scholar] [CrossRef]
- Adel, A.M.; El-Gendy, A.A.; Diab, M.A.; Abou-Zeid, R.E.; El-Zawawy, W.K.; Dufresne, A. Microfibrillated cellulose from agricultural residues. Part I: Papermaking application. Ind. Crops Prod. 2016, 93, 161–174. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Hassan, M.L. Physical and mechanical properties of microcrystalline cellulose prepared from agricultural residues. Carbohydr. Polym. 2007, 67, 1–10. [Google Scholar] [CrossRef]
- Shi, S.; Zhang, M.; Zhang, S.; Hou, W.; Yan, Z. Evolution of Waster Cotton Fiber Hydro-Char Physicochemical Structure during Hydrothermal Carbonation. Preprints 2017, 2017, 110149. [Google Scholar] [CrossRef]
- Ni, Z.-J.; Li, X.; Zhang, Y.-N.; Lou, B.-Y.; Pan, S.; Lv, Y.; Liu, D.-L. Micro-cellulose preparation method based on urea/naoh dissolved system. Univ. Politeh. Buchar. Sci. Bull. Ser. C Elect. Eng. Comput. Sci. 2017, 79, 45–54. [Google Scholar]
- Suryadi, H.; SUTRIYO, M.R.; LESTARI, Y.P.I. Potential of cellulase of Penicillium vermiculatum for preparation and characterization of microcrystalline cellulose produced from α-cellulose of kapok pericarpium (ceiba pentandra). Int. J. Appl. Pharm. 2019, 11, 92–97. [Google Scholar] [CrossRef]
- Hou, W.; Ling, C.; Shi, S.; Yan, Z. Preparation and characterization of microcrystalline cellulose from waste cotton fabrics by using phosphotungstic acid. Int. J. Biol. Macromol. 2019, 123, 363–368. [Google Scholar] [CrossRef]
- Chaerunisaa, A.Y.; Sriwidodo, S.; Abdassah, M. Microcrystalline Cellulose as Pharmaceutical Excipient. In Pharmaceutical Formulation Design-Recent Practices; IntechOpen: London, UK, 2019. [Google Scholar]
- Zugenmaier, P. Crystalline Cellulose and Derivatives: Characterization and Structures; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Khalil, H.A.; Bhat, A.; Yusra, A.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Ranby, B.G. Aqueous colloidal solutions of cellulose micelles. Acta Chem. Scand. 1949, 3, 649–650. [Google Scholar] [CrossRef] [Green Version]
- Mokhena, T.C.; John, M.J. Cellulose nanomaterials: New generation materials for solving global issues. Cellulose 2020, 27, 1149–1194. [Google Scholar] [CrossRef]
- Belali, N.G.; Chaerunisaa, A.Y.; Rusdiana, T. Isolation and Characterization of Microcrystalline Cellulose Derived from Plants as Excipient in Tablet: A Review. Indones. J. Pharm. 2019, 1, 55–61. [Google Scholar] [CrossRef]
- Colvin, J.R.; Beer, M. The formation of cellulose microfibrils in suspensions of Acetobacter xylinum. Can. J. Microbiol. 1960, 6, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, G. Hydrolysis of fibrous cotton and reprecipitated cellulose by cellulolytic enzymes from soil micro-organisms. Biochem. J. 1965, 95, 270–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heyn, A. The microcrystalline structure of cellulose in cell walls of cotton, ramie, and jute fibers as revealed by negative staining of sections. J. Cell Biol. 1966, 29, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Toshkov, T.S.; Gospodinov, N.R.; Vidimski, E.P. Method of Producing Microcrystalline Cellulose. U.S. Patent No. 3,954,727, 4 May 1976. [Google Scholar]
- Kobayashi, S.; Kashiwa, K.; Shimada, J.; Kawasaki, T.; Shoda, S.i. Enzymatic polymerization: The first in vitro synthesis of cellulose via nonbiosynthetic path catalyzed by cellulase. Macromol. Symp. 1992, 54, 509–518. [Google Scholar] [CrossRef]
- Revol, J.-F.; Godbout, L.; Gray, D. Solid self-assembled films of cellulose with chiral nematic order and optically variable properties. J. Pulp Paper Sci. 1998, 24, 146–149. [Google Scholar]
- Nakagaito, A.; Yano, H. The effect of morphological changes from pulp fiber towards nano-scale fibrillated cellulose on the mechanical properties of high-strength plant fiber based composites. Appl. Phys. A 2004, 78, 547–552. [Google Scholar] [CrossRef]
- Henriksson, M.; Henriksson, G.; Berglund, L.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Nyström, G.; Mihranyan, A.; Razaq, A.; Lindström, T.; Nyholm, L.; Strømme, M. A nanocellulose polypyrrole composite based on microfibrillated cellulose from wood. J. Phys. Chem. B 2010, 114, 4178–4182. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Chaussy, D.; Grosseau, P.; Beneventi, D. Use of microfibrillated cellulose/lignosulfonate blends as carbon precursors: Impact of hydrogel rheology on 3D printing. Ind. Eng. Chem. Res. 2015, 54, 10575–10582. [Google Scholar] [CrossRef]
- Alavi, M. Modifications of microcrystalline cellulose (MCC), nanofibrillated cellulose (NFC), and nanocrystalline cellulose (NCC) for antimicrobial and wound healing applications. e-Polymers 2019, 19, 103–119. [Google Scholar] [CrossRef]
- Nuryawan, A.; Abdullah, C.; Hazwan, C.M.; Olaiya, N.; Yahya, E.B.; Risnasari, I.; Masruchin, N.; Baharudin, M.; Khalid, H.; Abdul Khalil, H. Enhancement of Oil Palm Waste Nanoparticles on the Properties and Characterization of Hybrid Plywood Biocomposites. Polymers 2020, 12, 1007. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Shim, B.S.; Kim, H.S.; Lee, Y.-J.; Min, S.-K.; Jang, D.; Abas, Z.; Kim, J. Review of nanocellulose for sustainable future materials. Int. J. Prec. Eng. Manuf. Green Technol. 2015, 2, 197–213. [Google Scholar] [CrossRef] [Green Version]
- Soyekwo, F.; Zhang, Q.; Gao, R.; Qu, Y.; Lin, C.; Huang, X.; Zhu, A.; Liu, Q. Cellulose nanofiber intermediary to fabricate highly-permeable ultrathin nanofiltration membranes for fast water purification. J. Membr. Sci. 2017, 524, 174–185. [Google Scholar] [CrossRef]
- Lee, K.-Y. Nanocellulose and Sustainability: Production, Properties, Applications, and Case Studies; CRC Press: New York, NY, USA, 2018. [Google Scholar]
- Okahisa, Y.; Furukawa, Y.; Ishimoto, K.; Narita, C.; Intharapichai, K.; Ohara, H. Comparison of cellulose nanofiber properties produced from different parts of the oil palm tree. Carbohydr. Polym. 2018, 198, 313–319. [Google Scholar] [CrossRef]
- Doench, I.; Ahn Tran, T.; David, L.; Montembault, A.; Viguier, E.; Gorzelanny, C.; Sudre, G.; Cachon, T.; Louback-Mohamed, M.; Horbelt, N. Cellulose nanofiber-reinforced Chitosan hydrogel composites for intervertebral disc tissue repair. Biomimetics 2019, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Raza, Z.; Aslam, M.; Azeem, A.; Maqsood, H. Development and characterization of nano--crystalline cellulose incorporated poly (lactic acid) composite films. Mater. Werkst. 2019, 50, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Abraham, E.; Weber, D.E.; Sharon, S.; Lapidot, S.; Shoseyov, O. Multifunctional cellulosic scaffolds from modified cellulose nanocrystals. ACS Appl. Mater. Interfaces 2017, 9, 2010–2015. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yu, J.; Ma, J.; Wang, Z.; Fan, Y.; Zhou, X. High-yield preparation of cellulose nanofiber by small quantity acid assisted milling in glycerol. Cellulose 2019, 26, 3735–3745. [Google Scholar] [CrossRef]
- Jiang, F.; Hsieh, Y.-L. Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr. Polym. 2013, 95, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, K.; Hayashi, S.; Nishida, A.; Yamamoto, H.; Ducreux, J. Preparation of pure cellulose nanofiber via electrospinning. Text. Res. J. 2009, 79, 1396–1401. [Google Scholar] [CrossRef]
- Shamskar, K.R.; Heidari, H.; Rashidi, A. Preparation and evaluation of nanocrystalline cellulose aerogels from raw cotton and cotton stalk. Ind. Crops Prod. 2016, 93, 203–211. [Google Scholar] [CrossRef]
- Li, J.; Song, Z.; Li, D.; Shang, S.; Guo, Y. Cotton cellulose nanofiber-reinforced high density polyethylene composites prepared with two different pretreatment methods. Ind. Crops Prod. 2014, 59, 318–328. [Google Scholar] [CrossRef]
- Chin, S.F.; Jimmy, F.B.; Pang, S.C. Size controlled fabrication of cellulose nanoparticles for drug delivery applications. J. Drug Deliv. Sci. Technol. 2018, 43, 262–266. [Google Scholar] [CrossRef]
- Zhang, L.; Tsuzuki, T.; Wang, X. Preparation of cellulose nanofiber from softwood pulp by ball milling. Cellulose 2015, 22, 1729–1741. [Google Scholar] [CrossRef]
- Li, W.; Yue, J.; Liu, S. Preparation of nanocrystalline cellulose via ultrasound and its reinforcement capability for poly (vinyl alcohol) composites. Ultrason. Sonochem. 2012, 19, 479–485. [Google Scholar] [CrossRef]
- Cui, S.; Zhang, S.; Ge, S.; Xiong, L.; Sun, Q. Green preparation and characterization of size-controlled nanocrystalline cellulose via ultrasonic-assisted enzymatic hydrolysis. Ind. Crops Prod. 2016, 83, 346–352. [Google Scholar] [CrossRef]
- Hietala, M.; Rollo, P.; Kekäläinen, K.; Oksman, K. Extrusion processing of green biocomposites: Compounding, fibrillation efficiency, and fiber dispersion. J. Appl. Polym. Sci. 2014, 131, 39981. [Google Scholar] [CrossRef]
- Istomin, A.; Demina, T.; Subcheva, E.; Akopova, T.; Zelenetskii, A. Nanocrystalline cellulose from flax stalks: Preparation, structure, and use. Fibre Chem. 2016, 48, 199–201. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Montaser, A.; Li, S. Effect of cellulose nanocrystals on scaffolds comprising chitosan, alginate and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2019, 121, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Oh, D.X.; Choy, S.; Nguyen, H.-L.; Cha, H.J.; Hwang, D.S. 3D cellulose nanofiber scaffold with homogeneous cell population and long-term proliferation. Cellulose 2018, 25, 7299–7314. [Google Scholar] [CrossRef]
- Gu, H.; Gao, X.; Zhang, H.; Chen, K.; Peng, L. Fabrication and characterization of cellulose nanoparticles from maize stalk pith via ultrasonic-mediated cationic etherification. Ultrason. Sonochem. 2019, 66, 104932. [Google Scholar] [CrossRef]
- Prud’homme, R.K.; Feng, J.; Ristroph, K.D.; LU, H.; Zhang, Y.; Mcmanus, S.A.; Pagels, R.F. Cellulosic Polymer Nanoparticles and Methods of Forming Them. US Patent App. 16/816,241, 2 July 2020. [Google Scholar]
- Turbak, A.F.; Snyder, F.W.; Sandberg, K.R. Microfibrillated cellulose, a new cellulose product: Properties, uses, and commercial potential. J. Appl. Polym. Sci. Appl. Polym. Symp. 1983, 37. Available online: https://www.osti.gov/biblio/5062478-microfibrillated-cellulose-new-cellulose-product-properties-uses-commercial-potential (accessed on 1 January 1983).
- Azizi Samir, M.A.S.; Alloin, F.; Sanchez, J.-Y.; El Kissi, N.; Dufresne, A. Preparation of cellulose whiskers reinforced nanocomposites from an organic medium suspension. Macromolecules 2004, 37, 1386–1393. [Google Scholar] [CrossRef]
- Svagan, A.J.; Azizi Samir, M.A.; Berglund, L.A. Biomimetic polysaccharide nanocomposites of high cellulose content and high toughness. Biomacromolecules 2007, 8, 2556–2563. [Google Scholar] [CrossRef]
- Henriksson, M.; Berglund, L.A.; Isaksson, P.; Lindstrom, T.; Nishino, T. Cellulose nanopaper structures of high toughness. Biomacromolecules 2008, 9, 1579–1585. [Google Scholar] [CrossRef]
- Fang, B.; Wan, Y.-Z.; Tang, T.-T.; Gao, C.; Dai, K.-R. Proliferation and osteoblastic differentiation of human bone marrow stromal cells on hydroxyapatite/bacterial cellulose nanocomposite scaffolds. Tissue Eng. Part A 2009, 15, 1091–1098. [Google Scholar] [CrossRef]
- Rosa, M.; Medeiros, E.; Malmonge, J.; Gregorski, K.; Wood, D.; Mattoso, L.; Glenn, G.; Orts, W.; Imam, S. Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Crotogino, R. NanoCellulose. In Proceedings of the International Symposium on Assessing the Economic Impact of Nanotechnology, Washington, DC, USA, 27–28 March 2012. [Google Scholar]
- Dugan, J.M.; Gough, J.E.; Eichhorn, S.J. Bacterial cellulose scaffolds and cellulose nanowhiskers for tissue engineering. Nanomedicine 2013, 8, 287–298. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, Q.; Guo, W.; Terrell, L.; Qureshi, A.T.; Hayes, D.J.; Wu, Q. Electrospun bio-nanocomposite scaffolds for bone tissue engineering by cellulose nanocrystals reinforcing maleic anhydride grafted PLA. ACS Appl. Mater. Interfaces 2013, 5, 3847–3854. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Shi, K.; Zhitomirsky, I.; Cranston, E.D. Cellulose nanocrystal aerogels as universal 3D lightweight substrates for supercapacitor materials. Adv. Mater. 2015, 27, 6104–6109. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, F.; Grénman, H.; Spoljaric, S.; Seppälä, J.; Eriksson, J.E.; Willför, S.; Xu, C. Development of nanocellulose scaffolds with tunable structures to support 3D cell culture. Carbohydr. Polym. 2016, 148, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Li, V.C.-F.; Dunn, C.K.; Zhang, Z.; Deng, Y.; Qi, H.J. Direct ink write (DIW) 3D printed cellulose nanocrystal aerogel structures. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Apelgren, P.; Karabulut, E.; Amoroso, M.; Mantas, A.; Martínez Ávila, H.c.; Kölby, L.; Kondo, T.; Toriz, G.; Gatenholm, P. In vivo human cartilage formation in three-dimensional bioprinted constructs with a novel bacterial nanocellulose bioink. ACS Biomater. Sci. Eng. 2019, 5, 2482–2490. [Google Scholar] [CrossRef]
- Bukhari, N.; Joseph, J.P.; Hussain, J.; Adeeb, M.A.M.; Wakim, M.J.Y.; Yahya, E.B.; Arif, A.; Saleem, A.; Sharif, N. Prevalence of Human Papilloma Virus Sub Genotypes following Head and Neck Squamous Cell Carcinomas in Asian Continent, A Systematic Review Article. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 3269–3277. [Google Scholar] [CrossRef] [Green Version]
- Surya, I.; Olaiya, N.; Rizal, S.; Zein, I.; Sri Asprila, N.; Hasan, M.; Yahya, E.B.; Sadasivuni, K.; Abdul Khalil, H. Plasticizer Enhancement on the Miscibility and Thermomechanical Properties of Polylactic Acid-Chitin-Starch Composites. Polymers 2020, 12, 115. [Google Scholar] [CrossRef] [Green Version]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef]
- Gutiérrez-Hernández, J.M.; Escobar-García, D.M.; Escalante, A.; Flores, H.; González, F.J.; Gatenholm, P.; Toriz, G. In vitro evaluation of osteoblastic cells on bacterial cellulose modified with multi-walled carbon nanotubes as scaffold for bone regeneration. Mater. Sci. Eng. C 2017, 75, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Feiner, R.; Shapira, A.; Dvir, T. Scaffolds for tissue engineering of functional cardiac muscle. In Handbook of Tissue Engineering Scaffolds: Volume One; Elsevier: London, UK, 2019; pp. 685–703. [Google Scholar]
- Bölgen, N. Electrospun materials for bone and tendon/ligament tissue engineering. In Electrospun Materials for Tissue Engineering and Biomedical Applications; Elsevier: London, UK, 2017; pp. 233–260. [Google Scholar]
- Domingues, R.M.; Chiera, S.; Gershovich, P.; Motta, A.; Reis, R.L.; Gomes, M.E. Fabrication of anisotropically aligned nanofibrous scaffolds based on natural/synthetic polymer blends reinforced with cellulose nanocrystals for tendon tissue engineering. Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011. [Google Scholar] [CrossRef]
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue engineering and regenerative medicine: History, progress, and challenges. Ann. Rev. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef] [PubMed]
- Olaiya, N.G.; Nuryawan, A.; Oke, P.K.; Abdul Khalil, H.; Rizal, S.; Mogaji, P.; Sadiku, E.; Suprakas, S.; Farayibi, P.K.; Ojijo, V. The role of two-step blending in the properties of starch/chitin/polylactic acid biodegradable composites for biomedical applications. Polymers 2020, 12, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold techniques and designs in tissue engineering functions and purposes: A review. Adv. Mater. Sci. Eng. 2019, 2019. [Google Scholar] [CrossRef] [Green Version]
- Venkatesan, J.; Bhatnagar, I.; Kim, S.-K. Chitosan-alginate biocomposite containing fucoidan for bone tissue engineering. Mar. Drugs 2014, 12, 300–316. [Google Scholar] [CrossRef]
- Teimouri, A.; Azadi, M.; Emadi, R.; Lari, J.; Chermahini, A.N. Preparation, characterization, degradation and biocompatibility of different silk fibroin based composite scaffolds prepared by freeze-drying method for tissue engineering application. Polym. Degrad. Stab. 2015, 121, 18–29. [Google Scholar] [CrossRef]
- Jafari, H.; Shahrousvand, M.; Kaffashi, B. Reinforced poly (ε-caprolactone) bimodal foams via phospho-calcified cellulose nanowhisker for osteogenic differentiation of human mesenchymal stem cells. ACS Biomater. Sci. Eng. 2018, 4, 2484–2493. [Google Scholar] [CrossRef]
- Annabi, N.; Fathi, A.; Mithieux, S.M.; Martens, P.; Weiss, A.S.; Dehghani, F. The effect of elastin on chondrocyte adhesion and proliferation on poly (ɛ-caprolactone)/elastin composites. Biomaterials 2011, 32, 1517–1525. [Google Scholar] [CrossRef]
- Rodríguez, K.; Sundberg, J.; Gatenholm, P.; Renneckar, S. Electrospun nanofibrous cellulose scaffolds with controlled microarchitecture. Carbohydr. Polym. 2014, 100, 143–149. [Google Scholar] [CrossRef]
- Mi, H.-Y.; Jing, X.; Salick, M.R.; Cordie, T.M.; Turng, L.-S. Carbon nanotube (CNT) and nanofibrillated cellulose (NFC) reinforcement effect on thermoplastic polyurethane (TPU) scaffolds fabricated via phase separation using dimethyl sulfoxide (DMSO) as solvent. J. Mech. Behav. Biomed. Mater. 2016, 62, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Tang, A.; Li, J.; Li, J.; Zhao, S.; Liu, W.; Liu, T.; Wang, J.; Liu, Y. Nanocellulose/PEGDA aerogel scaffolds with tunable modulus prepared by stereolithography for three-dimensional cell culture. J. Biomater. Sci. Polym. Ed. 2019, 30, 797–814. [Google Scholar] [CrossRef]
- Salmoria, G.V.; Klauss, P.; Paggi, R.A.; Kanis, L.A.; Lago, A. Structure and mechanical properties of cellulose based scaffolds fabricated by selective laser sintering. Polym. Test. 2009, 28, 648–652. [Google Scholar] [CrossRef]
- Kolan, K.C.; Li, W.; Althage, R.; Semon, J.A.; Day, D.E.; Leu, M.C. Solvent and melt based extrusion 3D printing of polycaprolactone bioactive glass composite for tissue engineering. In Proceedings of the 3rd International Conference on Progress in Additive Manufacturing, Singapore, 14–17 May 2018. [Google Scholar]
- Li, L.; Zhu, Y.; Yang, J. 3D bioprinting of cellulose with controlled porous structures from NMMO. Mater. Lett. 2018, 210, 136–138. [Google Scholar] [CrossRef]
- Alemán--Domínguez, M.E.; Giusto, E.; Ortega, Z.; Tamaddon, M.; Benítez, A.N.; Liu, C. Three--dimensional printed polycaprolactone--microcrystalline cellulose scaffolds. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 521–528. [Google Scholar] [CrossRef]
- Huang, A.; Jiang, Y.; Napiwocki, B.; Mi, H.; Peng, X.; Turng, L.-S. Fabrication of poly (ε-caprolactone) tissue engineering scaffolds with fibrillated and interconnected pores utilizing microcellular injection molding and polymer leaching. RSC Adv. 2017, 7, 43432–43444. [Google Scholar] [CrossRef] [Green Version]
- Kanimozhi, K.; Basha, S.K.; Kumari, V.S.; Kaviyarasu, K. Development of biomimetic hybrid porous scaffold of chitosan/polyvinyl alcohol/carboxymethyl cellulose by freeze-dried and salt leached technique. J. Nanosci. Nanotechnol. 2018, 18, 4916–4922. [Google Scholar] [CrossRef]
- Chen, G.; Ushida, T.; Tateishi, T. Development of biodegradable porous scaffolds for tissue engineering. Mater. Sci. Eng. C 2001, 17, 63–69. [Google Scholar] [CrossRef]
- Zubairi, S.I. Porous three dimensional (3-D) scaffolds of poly (3-hydroxybutyric acid)(PHB) and poly (3-hydroxybutyric-co-3-hydroxyvaleric acid)(PHBV): Determination of salt leaching efficiency of solvent-casting particulate-leaching (SCPL). Adv. Environ. Biol. 2014, 8, 925–932. [Google Scholar]
- Khan, S.; Ul-Islam, M.; Ullah, M.W.; Ikram, M.; Subhan, F.; Kim, Y.; Jang, J.H.; Yoon, S.; Park, J.K. Engineered regenerated bacterial cellulose scaffolds for application in in vitro tissue regeneration. RSC Adv. 2015, 5, 84565–84573. [Google Scholar] [CrossRef]
- Abdelaal, O.A.; Darwish, S.M. Review of rapid prototyping techniques for tissue engineering scaffolds fabrication. In Characterization and Development of Biosystems and Biomaterials; Springer: Berlin/Heidelberg, Germany, 2013; pp. 33–54. [Google Scholar]
- Zhong, X.; Dehghani, F. Fabrication of biomimetic poly (propylene carbonate) scaffolds by using carbon dioxide as a solvent, monomer and foaming agent. Green Chem. 2012, 14, 2523–2533. [Google Scholar] [CrossRef]
- Li, Z.; Xie, M.-B.; Li, Y.; Ma, Y.; Li, J.-S.; Dai, F.-Y. Recent progress in tissue engineering and regenerative medicine. J. Biomater. Tissue Eng. 2016, 6, 755–766. [Google Scholar] [CrossRef]
- He, X.; Xiao, Q.; Lu, C.; Wang, Y.; Zhang, X.; Zhao, J.; Zhang, W.; Zhang, X.; Deng, Y. Uniaxially aligned electrospun all-cellulose nanocomposite nanofibers reinforced with cellulose nanocrystals: Scaffold for tissue engineering. Biomacromolecules 2014, 15, 618–627. [Google Scholar] [CrossRef]
- Liu, C.; Xia, Z.; Czernuszka, J. Design and development of three-dimensional scaffolds for tissue engineering. Chem. Eng. Res. Des. 2007, 85, 1051–1064. [Google Scholar] [CrossRef]
- Lu, T.; Li, Y.; Chen, T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int. J. Nanomed. 2013, 8, 337–350. [Google Scholar] [CrossRef] [Green Version]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Rimell, J.T.; Marquis, P.M. Selective laser sintering of ultra high molecular weight polyethylene for clinical applications. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Japn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2000, 53, 414–420. [Google Scholar] [CrossRef]
- Shuai, C.; Yuan, X.; Yang, W.; Peng, S.; He, C.; Feng, P.; Qi, F.; Wang, G. Cellulose nanocrystals as biobased nucleation agents in poly-l-lactide scaffold: Crystallization behavior and mechanical properties. Polym. Test. 2020, 85, 106458. [Google Scholar] [CrossRef]
- Zhang, B.; Cristescu, R.; Chrisey, D.B.; Narayan, R.J. Solvent-based Extrusion 3D Printing for the Fabrication of Tissue Engineering Scaffolds. Int. J. Bioprinting 2020, 6, 211. [Google Scholar] [CrossRef]
- Vaezi, M.; Yang, S. A novel bioactive PEEK/HA composite with controlled 3D interconnected HA network. Int. J. Bioprinting 2015, 1, 66–76. [Google Scholar] [CrossRef]
- Lu, X.; Lee, Y.; Yang, S.; Hao, Y.; Evans, J.R.; Parini, C.G. Fine lattice structures fabricated by extrusion freeforming: Process variables. J. Mater. Proc. Technol. 2009, 209, 4654–4661. [Google Scholar] [CrossRef]
- Wang, M.; Favi, P.; Cheng, X.; Golshan, N.H.; Ziemer, K.S.; Keidar, M.; Webster, T.J. Cold atmospheric plasma (CAP) surface nanomodified 3D printed polylactic acid (PLA) scaffolds for bone regeneration. Acta Biomater. 2016, 46, 256–265. [Google Scholar] [CrossRef]
- Wu, Z.; Su, X.; Xu, Y.; Kong, B.; Sun, W.; Mi, S. Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Molino, B.Z.; Wang, X.; Cheng, F.; Xu, W.; Molino, P.; Bacher, M.; Su, D.; Rosenau, T.; Willför, S. 3D printing of nanocellulose hydrogel scaffolds with tunable mechanical strength towards wound healing application. J. Mater. Chem. B 2018, 6, 7066–7075. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Salgado, A.; Gomes, M.E.; Chou, A.; Coutinho, O.; Reis, R.; Hutmacher, D. Preliminary study on the adhesion and proliferation of human osteoblasts on starch-based scaffolds. Mater. Sci. Eng. C 2002, 20, 27–33. [Google Scholar] [CrossRef]
- Banoriya, D.; Purohit, R.; Dwivedi, R. Modern trends in rapid prototyping for biomedical applications. Mater. Today Proc. 2015, 2, 3409–3418. [Google Scholar] [CrossRef]
- Hoque, M.E.; Chuan, Y.L.; Pashby, I. Extrusion based rapid prototyping technique: An advanced platform for tissue engineering scaffold fabrication. Biopolymers 2012, 97, 83–93. [Google Scholar] [CrossRef]
- Kim, G.H.; Ahn, S.H.; Lee, H.J.; Lee, S.; Cho, Y.; Chun, W. A new hybrid scaffold using rapid prototyping and electrohydrodynamic direct writing for bone tissue regeneration. J. Mater. Chem. 2011, 21, 19138–19143. [Google Scholar] [CrossRef]
- Seunarine, K.; Gadegaard, N.; Tormen, M.; Meredith, D.; Riehle, M.; Wilkinson, C. 3D polymer scaffolds for tissue engineering. Fut. Med. 2006, 1, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Mondschein, R.J.; Kanitkar, A.; Williams, C.B.; Verbridge, S.S.; Long, T.E. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials 2017, 140, 170–188. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Lin, L.; Zhan, S. Preparation and properties of cellulose nanocrystals, gelatin, hyaluronic acid composite hydrogel as wound dressing. J. Biomater. Sci. Poly. Ed. 2019, 30, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Ul-Islam, M.; Subhan, F.; Islam, S.U.; Khan, S.; Shah, N.; Manan, S.; Ullah, M.W.; Yang, G. Development of three-dimensional bacterial cellulose/chitosan scaffolds: Analysis of cell-scaffold interaction for potential application in the diagnosis of ovarian cancer. Int. J. Biol. Macromol. 2019, 137, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.R.; Sanchez-Martinez, C.; Strømme, M.; Ferraz, N. In vitro biological responses to nanofibrillated cellulose by human dermal, lung and immune cells: Surface chemistry aspect. Part. Fibre Toxicol. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ninan, N.; Muthiah, M.; Park, I.-K.; Elain, A.; Thomas, S.; Grohens, Y. Pectin/carboxymethyl cellulose/microfibrillated cellulose composite scaffolds for tissue engineering. Carbohydr. Polym. 2013, 98, 877–885. [Google Scholar] [CrossRef]
- Courtenay, J.C.; Johns, M.A.; Galembeck, F.; Deneke, C.; Lanzoni, E.M.; Costa, C.A.; Scott, J.L.; Sharma, R.I. Surface modified cellulose scaffolds for tissue engineering. Cellulose 2017, 24, 253–267. [Google Scholar] [CrossRef] [Green Version]
- Atila, D.; Keskin, D.; Tezcaner, A. Cellulose acetate based 3-dimensional electrospun scaffolds for skin tissue engineering applications. Carbohydr. Polym. 2015, 133, 251–261. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Ikram, M.; Islam, S.U.; Ullah, M.W.; Israr, M.; Jang, J.H.; Yoon, S.; Park, J.K. Preparation and structural characterization of surface modified microporous bacterial cellulose scaffolds: A potential material for skin regeneration applications in vitro and in vivo. Int. J. Biol. Macromol. 2018, 117, 1200–1210. [Google Scholar] [CrossRef]
- Czaja, W.; Kyryliouk, D.; DePaula, C.A.; Buechter, D.D. Oxidation of γ--irradiated microbial cellulose results in bioresorbable, highly conformable biomaterial. J. Appl. Polym. Sci. 2014, 131, 39995. [Google Scholar] [CrossRef]
- Edlund, U.; Lagerberg, T.; Ålander, E. Admicellar polymerization coating of CNF enhances integration in degradable nanocomposites. Biomacromolecules 2018, 20, 684–692. [Google Scholar] [CrossRef]
- Laurén, P. Biomedical Applications of Nanofibrillar Cellulose. Ph.D. Thesis, Helsinki University, Helsinki, Finland, 2018. [Google Scholar]
- Von Recum, H.A. From Biocompatibility to Immune Engineering. Exp. Biol. Med. 2016, 241, 889. [Google Scholar] [CrossRef] [Green Version]
- Togo, K.; Yamamoto, M.; Imai, M.; Akiyama, K.; Yamashita, A.C. Comparison of biocompatibility in cellulose triacetate dialysis membranes with homogeneous and asymmetric structures. Renal Replace. Ther. 2018, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Strätz, J.; Liedmann, A.; Heinze, T.; Fischer, S.; Groth, T. Effect of Sulfation Route and Subsequent Oxidation on Derivatization Degree and Biocompatibility of Cellulose Sulfates. Macromol. Biosc. 2019, 20, 1900403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramphul, H.; Gimié, F.; Andries, J.; Jhurry, D.; Bhaw-Luximon, A. Sugar-cane bagasse cellulose-based scaffolds promote multi-cellular interactions, angiogenesis and reduce inflammation for skin tissue regeneration. Int. J. Biol. Macromol. 2020, 157, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.A.A.R.; May, L.W.; Paterson, I.C.; John, J. OSC1: Assessment of Cytotoxicity of Microcrystalline Cellulose Reinforced Denture Base Resin. J. Indian Prosthodont. Soc. 2018, 18, S7. [Google Scholar] [CrossRef]
- Vartiainen, J.; Pöhler, T.; Sirola, K.; Pylkkänen, L.; Alenius, H.; Hokkinen, J.; Tapper, U.; Lahtinen, P.; Kapanen, A.; Putkisto, K. Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 2011, 18, 775–786. [Google Scholar] [CrossRef]
- He, X.; Fan, X.; Feng, W.; Chen, Y.; Guo, T.; Wang, F.; Liu, J.; Tang, K. Incorporation of microfibrillated cellulose into collagen-hydroxyapatite scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2018, 115, 385–392. [Google Scholar] [CrossRef]
- Kalita, R.D.; Nath, Y.; Ochubiojo, M.E.; Buragohain, A.K. Extraction and characterization of microcrystalline cellulose from fodder grass; Setaria glauca (L) P. Beauv, and its potential as a drug delivery vehicle for isoniazid, a first line antituberculosis drug. Coll. Surf. B Biointerfaces 2013, 108, 85–89. [Google Scholar] [CrossRef]
- Bajpai, S.; Chand, N.; Ahuja, S.; Roy, M. Vapor induced phase inversion technique to prepare chitosan/microcrystalline cellulose composite films: Synthesis, characterization and moisture absorption study. Cellulose 2015, 22, 3825–3837. [Google Scholar] [CrossRef]
- Singh, P.; Medronho, B.; dos Santos, T.; Nunes-Correia, I.; Granja, P.; Miguel, M.G.; Lindman, B. On the viability, cytotoxicity and stability of probiotic bacteria entrapped in cellulose-based particles. Food Hydrocoll. 2018, 82, 457–465. [Google Scholar] [CrossRef]
- Ogonowski, M.; Edlund, U.; Gorokhova, E.; Linde, M.; Ek, K.; Liewenborg, B.; Könnecke, O.; Navarro, J.R.; Breitholtz, M. Multi-level toxicity assessment of engineered cellulose nanofibrils in Daphnia magna. Nanotoxicology 2018, 12, 509–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Ji Gwak, E.; Jeong, S.; Lee, S.; Sim, W. Toxicity Evaluation of Cellulose Nanofibers (Cnfs) for Cosmetic Industry Application. J. Toxicol. Risk Assess 2019, 5, 29. [Google Scholar]
- Alexandrescu, L.; Syverud, K.; Gatti, A.; Chinga-Carrasco, G. Cytotoxicity tests of cellulose nanofibril-based structures. Cellulose 2013, 20, 1765–1775. [Google Scholar] [CrossRef]
- Coelho, C.C.; Michelin, M.; Cerqueira, M.A.; Gonçalves, C.; Tonon, R.V.; Pastrana, L.M.; Freitas-Silva, O.; Vicente, A.A.; Cabral, L.M.; Teixeira, J.A. Cellulose nanocrystals from grape pomace: Production, properties and cytotoxicity assessment. Carbohydr. Polym. 2018, 192, 327–336. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Fouda, A. Green approach for one-pot synthesis of silver nanorod using cellulose nanocrystal and their cytotoxicity and antibacterial assessment. Int. J. Biol. Macromol. 2018, 106, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, Y.; Wang, X.; Li, M.; Lei, H.; Xu, H. Cellulose nanocrystals prepared from wheat bran: Characterization and cytotoxicity assessment. Int. J. Biol. Macromol. 2019, 140, 225–233. [Google Scholar] [CrossRef]
- Nelson, C.; Khan, Y.; Laurencin, C.T. Nanofiber–microsphere (nano-micro) matrices for bone regenerative engineering: A convergence approach toward matrix design. Regen. Biomater. 2014, 1, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Lotfi, M.; Bagherzadeh, R.; Naderi-Meshkin, H.; Mahdipour, E.; Mafinezhad, A.; Sadeghnia, H.R.; Esmaily, H.; Maleki, M.; Hasssanzadeh, H.; Ghayaour-Mobarhan, M. Hybrid chitosan–ß-glycerol phosphate–gelatin nano-/micro fibrous scaffolds with suitable mechanical and biological properties for tissue engineering. Biopolymers 2016, 105, 163–175. [Google Scholar] [CrossRef]
- La Mantia, F.; Morreale, M.; Botta, L.; Mistretta, M.; Ceraulo, M.; Scaffaro, R. Degradation of polymer blends: A brief review. Polym. Degrad. Stab. 2017, 145, 79–92. [Google Scholar] [CrossRef]
- Kuzmina, O.; Heinze, T.; Wawro, D. Blending of cellulose and chitosan in alkyl imidazolium ionic liquids. ISRN Polym. Sci. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Rogovina, S.Z.; Vikhoreva, G.A. Polysaccharide-based polymer blends: Methods of their production. Glycoconj. J. 2006, 23, 611. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, S.; Nasreen, K.; Vijayalakshmi, K.; Govindarajan, C.; Sudha, P. Synthesis and Characterisation of Ternary Blends of Chitosan. Int. J. Chem. Res. 2011, 3, 27–32. [Google Scholar]
- HPS, A.K.; Saurabh, C.K.; Adnan, A.; Fazita, M.N.; Syakir, M.; Davoudpour, Y.; Rafatullah, M.; Abdullah, C.; Haafiz, M.; Dungani, R. A review on chitosan-cellulose blends and nanocellulose reinforced chitosan biocomposites: Properties and their applications. Carbohydr. Polym. 2016, 150, 216–226. [Google Scholar]
- Tuancharoensri, N.; Ross, G.M.; Mahasaranon, S.; Topham, P.D.; Ross, S. Ternary blend nanofibres of poly (lactic acid), polycaprolactone and cellulose acetate butyrate for skin tissue scaffolds: Influence of blend ratio and polycaprolactone molecular mass on miscibility, morphology, crystallinity and thermal properties. Polym. Int. 2017, 66, 1463–1472. [Google Scholar] [CrossRef]
- Khalili, S.; Khorasani, S.N.; Razavi, S.M.; Hashemibeni, B.; Tamayol, A. Nanofibrous scaffolds with biomimetic composition for skin regeneration. Appl. Biochem. Biotechnol. 2019, 187, 1193–1203. [Google Scholar] [CrossRef]
- Bajaj, P.; Schweller, R.M.; Khademhosseini, A.; West, J.L.; Bashir, R. 3D biofabrication strategies for tissue engineering and regenerative medicine. Ann. Rev. Biomed. Eng. 2014, 16, 247–276. [Google Scholar] [CrossRef] [Green Version]
- Nada, A.A.; Arul, M.R.; Ramos, D.M.; Kroneková, Z.; Mosnáček, J.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric formulations for wound healing. Polym. Adv. Technol. 2018, 29, 1815–1825. [Google Scholar] [CrossRef]
- Rusu, D.; Ciolacu, D.; Simionescu, B.C. Cellulose-based hydrogels in tissue engineering applications. Cell. Chem. Technol. 2019, 53, 907–923. [Google Scholar] [CrossRef]
- Müller, M.; Öztürk, E.; Arlov, Ø.; Gatenholm, P.; Zenobi-Wong, M. Alginate sulfate–nanocellulose bioinks for cartilage bioprinting applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Ghaemi, F.; Abdullah, L.C. Comparative study of cytotoxicity effect between cellulose nanocrystal and cellulose nanofiber. Adv. Nanobiotechnol. 2018, 1, 1–3. [Google Scholar]
- Roman, M. Toxicity of cellulose nanocrystals: A review. Ind. Biotechnol. 2015, 11, 25–33. [Google Scholar] [CrossRef]
- Blackstone, B.N.; Hahn, J.M.; McFarland, K.L.; DeBruler, D.M.; Supp, D.M.; Powell, H.M. Inflammatory response and biomechanical properties of coaxial scaffolds for engineered skin in vitro and post-grafting. Acta Biomater. 2018, 80, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.M.; Gomes, M.E.; Reis, R.L. The potential of cellulose nanocrystals in tissue engineering strategies. Biomacromolecules 2014, 15, 2327–2346. [Google Scholar] [CrossRef]
- Sivashanmugam, A.; Kumar, R.A.; Priya, M.V.; Nair, S.V.; Jayakumar, R. An overview of injectable polymeric hydrogels for tissue engineering. Eur. Polym. J. 2015, 72, 543–565. [Google Scholar] [CrossRef]
- Xu, D.; Fan, L.; Gao, L.; Xiong, Y.; Wang, Y.; Ye, Q.; Yu, A.; Dai, H.; Yin, Y.; Cai, J. Micro-nanostructured polyaniline assembled in cellulose matrix via interfacial polymerization for applications in nerve regeneration. ACS Appl. Mater. Interfaces 2016, 8, 17090–17097. [Google Scholar] [CrossRef]
- Piras, C.C.; Fernández-Prieto, S.; De Borggraeve, W.M. Nanocellulosic materials as bioinks for 3D bioprinting. Biomater. Sci. 2017, 5, 1988–1992. [Google Scholar] [CrossRef]
- Markstedt, K.; Escalante, A.; Toriz, G.; Gatenholm, P. Biomimetic inks based on cellulose nanofibrils and cross-linkable xylans for 3D printing. ACS Appl. Mater. Interfaces 2017, 9, 40878–40886. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, Z.Y.W.; Wenger, A.C.; Tam, K.C.; Tang, X.S. 3D bioprinting of liver-mimetic construct with alginate/cellulose nanocrystal hybrid bioink. Bioprinting 2018, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Johnen, C.; Steffen, I.; Beichelt, D.; Bräutigam, K.; Witascheck, T.; Toman, N.; Moser, V.; Ottomann, C.; Hartmann, B.; Gerlach, J. Culture of subconfluent human fibroblasts and keratinocytes using biodegradable transfer membranes. Burns 2008, 34, 655–663. [Google Scholar] [CrossRef]
- Sanchavanakit, N.; Sangrungraungroj, W.; Kaomongkolgit, R.; Banaprasert, T.; Pavasant, P.; Phisalaphong, M. Growth of human keratinocytes and fibroblasts on bacterial cellulose film. Biotechnol. Prog. 2006, 22, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Wang, J.; Chen, H.; Shi, X.; Wang, X.; Zhu, Y.; Tan, Z. The topography of fibrous scaffolds modulates the paracrine function of Ad-MSCs in the regeneration of skin tissues. Biomater. Sci. 2019, 7, 4248–4259. [Google Scholar] [CrossRef] [PubMed]
- Rees, A.; Powell, L.C.; Chinga-Carrasco, G.; Gethin, D.T.; Syverud, K.; Hill, K.E.; Thomas, D.W. 3D bioprinting of carboxymethylated-periodate oxidized nanocellulose constructs for wound dressing applications. BioMed Res. Int. 2015, 2015, 925757. [Google Scholar] [CrossRef] [Green Version]
- Cecen, B.; Kozaci, L.D.; Yuksel, M.; Ustun, O.; Ergur, B.U.; Havitcioglu, H. Biocompatibility and biomechanical characteristics of loofah based scaffolds combined with hydroxyapatite, cellulose, poly-l-lactic acid with chondrocyte-like cells. Mater. Sci. Eng. C 2016, 69, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Torres--Rendon, J.G.; Femmer, T.; De Laporte, L.; Tigges, T.; Rahimi, K.; Gremse, F.; Zafarnia, S.; Lederle, W.; Ifuku, S.; Wessling, M. Bioactive gyroid scaffolds formed by sacrificial templating of nanocellulose and nanochitin hydrogels as instructive platforms for biomimetic tissue engineering. Adv. Mater. 2015, 27, 2989–2995. [Google Scholar] [CrossRef]
- Shi, Z.; Gao, H.; Feng, J.; Ding, B.; Cao, X.; Kuga, S.; Wang, Y.; Zhang, L.; Cai, J. In situ synthesis of robust conductive cellulose/polypyrrole composite aerogels and their potential application in nerve regeneration. Angew. Chem. Int. Ed. 2014, 53, 5380–5384. [Google Scholar] [CrossRef]
- Sun, D.; Liu, W.; Tang, A.; Guo, F.; Xie, W. A new PEGDA/CNF aerogel-wet hydrogel scaffold fabricated by a two-step method. Soft Matter 2019, 15, 8092–8101. [Google Scholar] [CrossRef]
- Innala, M.; Riebe, I.; Kuzmenko, V.; Sundberg, J.; Gatenholm, P.; Hanse, E.; Johannesson, S. 3D Culturing and differentiation of SH-SY5Y neuroblastoma cells on bacterial nanocellulose scaffolds. Artif. Cells Nanomed. Biotechnol. 2014, 42, 302–308. [Google Scholar] [CrossRef]
- Jonsson, M.; Brackmann, C.; Puchades, M.; Brattås, K.; Ewing, A.; Gatenholm, P.; Enejder, A. Neuronal networks on nanocellulose scaffolds. Tissue Eng. Part C Methods 2015, 21, 1162–1170. [Google Scholar] [CrossRef]
- Pircher, N.; Fischhuber, D.; Carbajal, L.; Strauß, C.; Nedelec, J.M.; Kasper, C.; Rosenau, T.; Liebner, F. Preparation and reinforcement of dual--porous biocompatible cellulose scaffolds for tissue engineering. Macromol. Mater. Eng. 2015, 300, 911–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, J.R.; Wennmalm, S.; Godfrey, J.; Breitholtz, M.; Edlund, U. Luminescent nanocellulose platform: From controlled graft block copolymerization to biomarker sensing. Biomacromolecules 2016, 17, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, N.; Okkelman, I.A.; Timashev, P.; Gromovykh, T.I.; Papkovsky, D.B.; Dmitriev, R.I. Cellulose-based scaffolds for fluorescence lifetime imaging-assisted tissue engineering. Acta Biomater. 2018, 80, 85–96. [Google Scholar] [CrossRef]
- Kim, K.O.; Kim, G.J.; Kim, J.H. A cellulose/β-cyclodextrin nanofiber patch as a wearable epidermal glucose sensor. RSC Adv. 2019, 9, 22790–22794. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Cheng, W.; Liu, K.; Chen, L.; Huang, Y.; Wang, X.; Lv, Z.; He, J.; Li, C. Reinforced collagen with oxidized microcrystalline cellulose shows improved hemostatic effects. Carbohydr. Polym. 2017, 165, 30–38. [Google Scholar] [CrossRef]
- Quero, F.; Padilla, C.; Campos, V.; Luengo, J.; Caballero, L.; Melo, F.; Li, Q.; Eichhorn, S.J.; Enrione, J. Stress transfer and matrix-cohesive fracture mechanism in microfibrillated cellulose-gelatin nanocomposite films. Carbohydr. Polym. 2018, 195, 89–98. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nip, J.; Kalajzic, I.; Kumbar, S.G. Micro-nanostructures of cellulose-collagen for critical sized bone defect healing. Macromol. Biosci. 2018, 18, 1700263. [Google Scholar] [CrossRef]
- Wei, D.; Liu, Q.; Liu, Z.; Liu, J.; Zheng, X.; Pei, Y.; Tang, K. Modified nano microfibrillated cellulose/carboxymethyl chitosan composite hydrogel with giant network structure and quick gelation formability. Int. J. Biol. Macromol. 2019, 135, 561–568. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, C.; Liu, Y.; Jiang, W.; Han, G. Preparation and characterization of composite scaffold of alginate and cellulose nanofiber from ramie. Text. Res. J. 2019, 89, 3260–3268. [Google Scholar] [CrossRef]
- Lohrasbi, S.; Mirzaei, E.; Karimizade, A.; Takallu, S.; Rezaei, A. Collagen/cellulose nanofiber hydrogel scaffold: Physical, mechanical and cell biocompatibility properties. Cellulose 2020, 27, 927–940. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Si, J.; Cui, Z.; Peng, K. Morphological, mechanical and thermal properties of poly (lactic acid)(PLA)/cellulose nanofibrils (CNF) composites nanofiber for tissue engineering. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 207–215. [Google Scholar] [CrossRef]







| Properties | Plants Cellulose | Bacterial Cellulose | References |
|---|---|---|---|
| Purity | Moderate to low | High | [31] |
| Crystallinity degree | 54–88% | 65–79% | [32] |
| Degree of polymerization | Ranged from 500–15,000 | 800–10,000 | [33] |
| Availability | Highly available | Limited | [34] |
| Industrial-scale production | Limited | Very limited | [34] |
| Material | Preparation Process | Particle Size | References |
|---|---|---|---|
| MFC | Acid hydrolysis | 9–16 μm | [5] |
| MCC | Treatment of urea/NaOH | 0.05–0.6 μm | [52] |
| MCC | Enzymatic hydrolysis | 0.01–200 μm | [53] |
| MCC | Catalytic hydrolysis | 8.68–31.1 μm | [54] |
| MFC | Alkali degumming process | 0.25–0.30 µm | [47] |
| MCC | Acid hydrolysis | 20 to 374 µm | [43] |
| Researchers | Progress | References |
|---|---|---|
| Payen (1839) | The first-time isolation of cellulose as the principal constituent of wood. | [56] |
| Schonbein (1845) | The first invention of cellulose esters. | [57] |
| Ranby (1949) | The first production of microcellulose and nanocrystals with acid hydrolysis of cellulose fibres dispersed in water. | [58] |
| Ranby (1951) | First synthesised colloidal suspensions of cellulose with acid-catalysed degradation of cellulose fibres. | [23] |
| Mukherjee et al. (1952) | First TEM images of cellulose materials. | [59] |
| Battista and Smith (1955) | Microcrystalline cellulose first discovery. | [60] |
| Colvin et al. (1960) | Formation of micro-fibrillated cellulose in suspensions of Acetobacter xylinum. | [61] |
| Halliwell et al. (1965) | Soil micro-organisms cellulolytic enzymes to re-precipitate cellulose and preparing it by hydrolysis of fibrous cotton. | [62] |
| Heyn et al. (1966) | Extensively study of the microcrystalline structure of cellulose in cell walls of plants fibres as revealed by negative staining of sections. | [63] |
| Toshkov et al. (1976) | Development of various method to produce microcrystalline cellulose. | [64] |
| Kobayashi and Shoda (1992) | First full chemically synthesised cellulose (non-biosynthetic path). | [65] |
| Revol et al. (1998) | Development of cellulose-based solidified liquid crystals for various optical applications. | [66] |
| Nakagaito & Yano (2004) | Applying of cellulose microfibril for semi-structural applications | [67] |
| Kulpinski (2005), Viswanathan et al. (2006) | Electrospinning of pure cellulose. | [59] |
| Henriksson et al. (2007) | Preparation of micro-fibrillated cellulose nanofibres with an environmentally friendly method for enzyme-assisted. | [68] |
| Nyström et al. (2010) | Development of nanocellulose polypyrrole composite based on micro-fibrillated cellulose from wood. | [69] |
| Shao et al. (2015) | Use of micro-fibrillated cellulose/lignosulfonate blends hydrogel rheology on 3D printing. | [70] |
| Alavi et al. (2019) | Modifications of microcrystalline cellulose for antimicrobial and wound healing applications. | [71] |
| Material | Preparation Process | Particle Diameter | Reference |
|---|---|---|---|
| Cellulose nanofibre | Ball milling | 100 nm | [86] |
| Cellulose nanofibre | Electrospinning | 70 to 130 nm | [82] |
| Nanocrystalline cellulose | High-intensity ultrasonication | 10 and 20 nm | [87] |
| Cellulose nanofibre | Blending Method | 15–20 nm | [81] |
| Nanocrystalline cellulose | Acid hydrolysis | 10 nm | [88] |
| Cellulose nanofibre | Twin-Screw Extrusion | 30 nm | [89] |
| Nanocrystalline cellulose | Acid hydrolysis | 85 nm | [90] |
| Researchers | Progress | References |
|---|---|---|
| Ranby (1949) | The first production of microcellulose and nanocrystals with acid hydrolysis of cellulose fibres dispersed in water. | [58] |
| Turbak and Herrick (1983) | First isolation of nanofibrillated cellulose with mechanical homogenisation of wood. | [95] |
| Favier et al. (1995) | The first report demonstrating the reinforcing effect of cellulose nanocrystals. | [59] |
| Azizi Samir et al. (2004) | Isolation of cellulose whiskers reinforced nanocomposites from an organic medium suspension. | [96] |
| Svagan et al. (2007) | Preparation of cellulose nanofibres bio-foams from wood pulp-based on amylopectin-rich potato starch. | [97] |
| Henriksson et al. (2008) | Development of Nano-paper from cellulose nanofibre suspensions. | [98] |
| Fang et al. (2009) | Fabrication of hydroxyapatite/bacterial cellulose nanocomposite scaffolds for the cultivation of human bone marrow stromal cells. | [99] |
| Rosa et al. (2010) | Isolation and characterisation of cellulose nanofibre from coconut husk fibres. | [100] |
| Crotogino (2012) | First pilot plant for cellulose nanomaterials production by Innventia in Sweden. | [101] |
| Dugan et al. (2013) | Development of bacterial cellulose scaffolds and cellulose nanofibre for tissue engineering applications. | [102] |
| Zhou et al. (2013) | Development of electrospun cellulose nanocrystals-based scaffolds for bone tissue engineering, reinforcing maleic anhydride grafted PLA. | [103] |
| Yang et al. (2015) | Fabrication of cellulose nanocrystal-based aerogels as universal 3D lightweight substrates for supercapacitor materials. | [104] |
| Liu et al. (2016) | Development of nanocellulose scaffolds with tunable structures to support 3D cell culture | [105] |
| Li et al. (2017) | 3D printing of many aerogel structures from pure cellulose nanocrystal with direct ink writing technique. | [106] |
| Apelgren et al. (2019) | In vivo formation of human cartilage in 3D bio-printed constructs with a novel bacterial nanocellulose bio-ink. | [107] |
| Part A: Conventional Techniques for Scaffold Fabrication | |||
| Technique | Operational Condition | Principal | Reference |
| Freeze-drying | Pre-freezing −20 °C for 12 h, then freeze-drying at −80 °C for 48 h | Using aqueous crystals instead of an organic solvent and does not require high temperatures. | [120] |
| Solvent casting and practical leaching | 70 °C to dissolve the salts | Different solvents and salt particles used, which then evaporated leaching out the salts. | [121] |
| Gas foaming | CO2 at 65 bar, 70 °C, the processing time of 1 h | High temperature and organic solvents used in dissolving & inert gases to pressurise modelled it until it saturated or full of gas bubbles. | [122] |
| Electrospinning | High voltage power supply | Charged threads of polymeric solution or polymer melt are drawn by high voltage electricity. | [123] |
| Thermal-induced phase separation | Dissolved for 8 h at 70 °C with 300 rpm magnetic stirring, then water evaporation at 100 °C. | Alteration of temperature to force phase separation of the polymer solution, and then dried used to form a nanoscale fibrous network. | [124] |
| Part B: Rapid Prototyping Techniques for Scaffold Fabrication | |||
| Technique | Operational Condition | Principal | Reference |
| Stereolithography | UV-light (365 nm, 1500 W) for 40 s, then −80 °C for 12 h followed by freeze-drying at −68 °C for 48 h | Layer-by-layer printing of photosensitive liquid of polymer with an ultraviolet laser until a 3D scaffold fabricated. | [125] |
| Selective laser sintering | Laser scan speed 39.8 mm/s, laser power ranged from 1.5 to 3.5 W. | The selective laser used to sinter powdered material in thin layers until a 3D scaffold fabricated. | [126] |
| Solvent-based extrusion free-forming | Heating temperature of 170 °C and air pressure of 80 psi | Discharging of liquid to solid transition through solvent evaporation in the presence of a binder. | [127] |
| Bioprinting | 3D printing was at 70 °C, then freeze to −70 °C for 12 h followed by freeze-drying. | Fabrication of layer-by-layer, of selective powdered material, then taking out the unbound powder, yielding a complex 3D scaffold. | [128] |
| Fused deposition modelling | Printing temperature was 210 °C. | Layer-by-layer deposition of polymeric materials extruded through a nozzle to create 3D multiple layers scaffolds. | [129] |
| Injection moulding | At room temperature | Special moulding machine used to melt and inject the polymeric material into the mould, where it cools and solidifies into the final part. | [130] |
| Functionality | Conventional Techniques | Rapid Prototyping Techniques | Reference |
|---|---|---|---|
| Scaffold development time & computer aid | Relatively slow without computer aid | Rapid preparation with computer-aided development | [152] |
| Manpower or technicians requirements | Require technicians and more manpower needed | Minimize manpower due to computer-controlled fabrication | [147] |
| Scaffold homogeneity | Not able to develop homogeneous structures | Homogeneous structures can be easily developed | [153] |
| Control the internal microstructure of scaffolds | Difficult or able to control the internal structures | The internal structures can be easily controlled | [154] |
| Scaffold porosity | Irregular pores shape and insufficient interconnectivity | Regular and interconnected pores | [155] |
| Effects on scaffold cytotoxicity | More effect on cells due to solvent residue | Less effect on cells, no solvent residue | [153] |
| Ability to design accurate & desirable shapes | Difficult to prepare the desirable shape | Easy to prepare even complex shapes | [156] |
| Cost of production | High cost of production | Low cost of production | [141] |
| Material | Experiment | Conclusion | Reference |
|---|---|---|---|
| Microcrystalline Cellulose (MCC) | Cytotoxicity and viability evaluation. | No sign for cytotoxicity was observed. | [172] |
| micro fibrillated cellulose (NFC) | Viability and cytokine of mouse and human cells | Not cytotoxic and does not cause any effects to the cells. | [173] |
| MFC/ collagen–hydroxyapatite (Col/HA) composite | Viability and proliferation of cells with MTT assay | The composite has been not cytotoxic, biocompatible and safe to cells. | [174] |
| Microcrystalline cellulose | Haemolytic assay | No cytotoxicity was observed. | [175] |
| Microcrystalline cellulose/chitosan composite | Cytotoxicity, Thrombogenesis, and haemolytic evaluation. | The composite showed neither cytotoxicity nor thrombogenicity. | [176] |
| Cellulose-based particles | Viability of the probiotic bacteria assessment. | Generally presented a low toxicity profile to the cell line. | [177] |
| Cellulose nanofibres | Eco-toxicological and feeding experiments to Daphnia Magna. | Low toxic potential to filter-feeding organisms and low expected environmental risks. | [178] |
| Cellulose nanofibres | Cytotoxicity, skin, and eye irritation tests. | Significantly induced cytotoxicity But not induce skin and eye irritation on 3D models. | [179] |
| Cellulose nanofibrils | Cytotoxicity and viability of fibroblast cells | No, exert acute toxic phenomena on the cells was observed. | [180] |
| Cellulose nanocrystals | Cytotoxicity and viability evaluation. | No sign for cytotoxicity was observed. | [181] |
| Cellulose nanocrystal/silver nanorod | In vitro cytotoxicity of against multiple eukaryotic cells. | No effect on eukaryotic cells was observed. | [182] |
| Cellulose nanocrystals | Cytotoxicity and viability with the MTT assay. | Cell viability slightly decreased with increasing in CNC concentration | [183] |
| Material | Application & Advantages | Reference |
|---|---|---|
| Collagen/oxidized MCC | Microcellulose improved the haemostasis of the scaffolds without affecting its cytotoxicity. | [220] |
| MFC/gelatine nanocomposite films | Improved strength and flexibility of the films, which could be used in drug delivery. | [221] |
| Micro-Nano structures of cellulose-collagen | Significantly enhanced the uniform and distribution of cells, with good mechanical properties, may serve as an alternative material platform for bone regeneration. | [222] |
| MFC/carboxymethyl chitosan hydrogel | The strength, porosity and the work of fracture increased, providing a promising platform for tissue engineering scaffold. | [223] |
| Alginate/CNF scaffold | Use in tissue engineering. CNF enhances mechanical properties and makes it possible to tailored porosity and swelling behaviour. | [224] |
| Collagen/CNF hydrogel scaffold | The addition of CNF to collagen scaffold improved its mechanical properties with no effect on cell viability. | [225] |
| PLA/CNF composite membrane | CNF improved the crystalline ability of the membrane, thermal stability and mechanical properties. Hydrophilicity was also increased. | [226] |
| NCC/gelatine/hyaluronic acid composite hydrogel | NCC enhanced rheology and swelling results and the other properties. The cells attached, grew, and proliferated better than the control, giving the composite a great potential for the skin wound repair. | [157] |
| Double crosslinking CNF hydrogel scaffolds | Wound healing and tissue repair. Increase in the rigidity of scaffold enhances cell proliferation. | [149] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, H.P.S.A.; Jummaat, F.; Yahya, E.B.; Olaiya, N.G.; Adnan, A.S.; Abdat, M.; N. A. M., N.; Halim, A.S.; Kumar, U.S.U.; Bairwan, R.; et al. A Review on Micro- to Nanocellulose Biopolymer Scaffold Forming for Tissue Engineering Applications. Polymers 2020, 12, 2043. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092043
Khalil HPSA, Jummaat F, Yahya EB, Olaiya NG, Adnan AS, Abdat M, N. A. M. N, Halim AS, Kumar USU, Bairwan R, et al. A Review on Micro- to Nanocellulose Biopolymer Scaffold Forming for Tissue Engineering Applications. Polymers. 2020; 12(9):2043. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092043
Chicago/Turabian StyleKhalil, H. P. S. Abdul, Fauziah Jummaat, Esam Bashir Yahya, N. G. Olaiya, A. S. Adnan, Munifah Abdat, Nasir N. A. M., Ahmad Sukari Halim, U. Seeta Uthaya Kumar, Rahul Bairwan, and et al. 2020. "A Review on Micro- to Nanocellulose Biopolymer Scaffold Forming for Tissue Engineering Applications" Polymers 12, no. 9: 2043. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092043