Fabrication of Tizanidine Loaded Patches Using Flaxseed Oil and Coriander Oil as a Penetration Enhancer for Transdermal Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
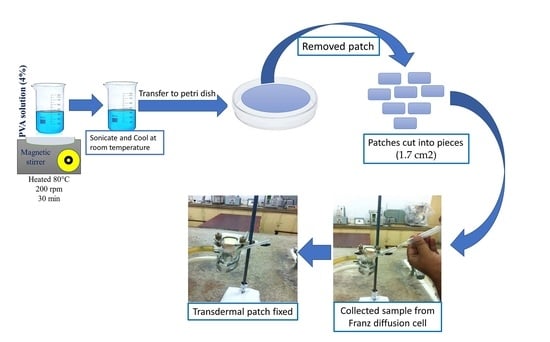
2.2. Fabrication of Transdermal Patches
2.2.1. Differential Scanning Calorimetry (DSC) Study
2.2.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3. Thickness, Weight Uniformity, and Efficiency of Patches
2.4. Percentage Moisture Uptake and Percentage Moisture Loss
2.5. Drug Content
2.6. In Vitro Drug Release Studies of the Prepared Patch
2.7. Drug Release Kinetics Mechanism
2.8. In Vitro Drug Permeation Study of Tizanidine
2.9. Stability Study
2.10. Skin Irritation Study
2.11. In Vivo Drug Release
2.12. Application of a Patch on Rabbit Skin for In Vivo Study
2.13. Extraction and Analysis of Tizanidine in Rabbit Plasma
2.14. Pharmacokinetics Analysis
3. Results
3.1. Physicochemical Evaluation of Patches
3.2. DSC Studies
3.3. FT-IR Studies
3.4. In Vitro Dissolution of Patches
3.5. Release Kinetics of Patches
3.6. Thickness, Weight, Folding Endurance, Moisture Uptake and Loss, Flatness, and Drug Content of Patches
3.7. Stability Study of the Patches
3.8. Skin Irritation Studies
3.9. In Vitro Permeation Study of Tizanidine by Using Flaxseed Oil and Coriander Oil as Permeation Enhancers
3.10. In Vivo Study of Tizanidine Transdermal Patches and Oral Tablets
3.11. Pharmacokinetics Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| F. Code | Drug Quantity | Polymer | Enhancer | Plasticizer Di-n-butyle Phthalate (%) | Ethanol (mL) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chitosan | Thiolated Chitosan | EC | PVP | Eudragit | PVP | Flaxseed Oil (%) | Corriander Oil (%) | ||||
| FTZN1 | 120 | 1 | 1 | ||||||||
| FTZN2 | 120 | 1.4 | 0.6 | ||||||||
| FTZN3 | 120 | 1.7 | 0.3 | ||||||||
| FTZN14 | 120 | 2:0 | 0.0 | ||||||||
| FTZN15 | 120 | 0.6 | 1.4 | ||||||||
| FTZN16 | 120 | 0.3 | 1.7 | ||||||||
| FTZN17 | 120 | 5 | 0 | ||||||||
| FTZN18 | 120 | 5 | 1 | ||||||||
| FTZN19 | 120 | 3 | 1 | ||||||||
| FTZN110 | 120 | 1 | 1 | ||||||||
| FTZN111 | 120 | 1 | 3 | ||||||||
| FTZN112 | 120 | 1 | 5 | ||||||||
| FTZN113 | 120 | 5 | 0 | ||||||||
| FTZN114 | 120 | 5 | 1 | ||||||||
| FTZN115 | 120 | 3 | 1 | ||||||||
| FTZN116 | 120 | 1 | 1 | ||||||||
| FTZN117 | 120 | 1 | 3 | ||||||||
| FTZN118 | 120 | 1 | 5 | ||||||||
| FTZN1E19 | 120 | 600 | 600 | 1 | 15 | 10 | |||||
| FTZN1E20 | 120 | 840 | 360 | 2 | 15 | 10 | |||||
| FTZN1E21 | 120 | 1020 | 180 | 3 | 15 | 10 | |||||
| FTZN1E22 | 120 | 1200 | 0.00 | 4 | 15 | 10 | |||||
| FTZN1E23 | 120 | 360 | 840 | 5 | 15 | 10 | |||||
| FTZN1E24 | 120 | 180 | 1020 | 10 | 15 | 10 | |||||
| FTZN1E25 | 120 | 600 | 600 | 1 | 15 | 10 | |||||
| FTZN1E26 | 120 | 840 | 360 | 2 | 15 | 10 | |||||
| FTZN1E27 | 120 | 1020 | 180 | 3 | 15 | 10 | |||||
| FTZN1E28 | 120 | 1200 | 0.00 | 4 | 15 | 10 | |||||
| FTZN1E29 | 120 | 360 | 840 | 5 | 15 | 10 | |||||
| FTZN1E30 | 120 | 180 | 1020 | 10 | 15 | 10 | |||||
| FTZN1E31 | 120 | 60 | 20 | 1 | 15 | 10 | |||||
| FTZN1E32 | 120 | 60 | 20 | 2 | 15 | 10 | |||||
| FTZN1E33 | 120 | 60 | 20 | 3 | 15 | 10 | |||||
| FTZN1E34 | 120 | 60 | 20 | 4 | 15 | 10 | |||||
| FTZN1E35 | 120 | 60 | 20 | 5 | 15 | 10 | |||||
| FTZN1E36 | 120 | 60 | 20 | 10 | 15 | 10 | |||||
| FTZN1E37 | 120 | 60 | 20 | 1 | 15 | 10 | |||||
| FTZN1E38 | 120 | 60 | 20 | 2 | 15 | 10 | |||||
| FTZN1E39 | 120 | 60 | 20 | 3 | 15 | 10 | |||||
| FTZN1E40 | 120 | 60 | 20 | 4 | 15 | 10 | |||||
| FTZN1E41 | 120 | 60 | 20 | 5 | 15 | 10 | |||||
| FTZN1E42 | 120 | 60 | 20 | 10 | 15 | 10 | |||||
| FTZN1E43 | 120 | 40 | 40 | 1 | 15 | 10 | |||||
| FTZN1E44 | 120 | 40 | 40 | 2 | 15 | 10 | |||||
| FTZN1E45 | 120 | 40 | 40 | 3 | 15 | 10 | |||||
| FTZN1E46 | 120 | 40 | 40 | 4 | 15 | 10 | |||||
| FTZN1E47 | 120 | 40 | 40 | 5 | 15 | 10 | |||||
| FTZN1E48 | 120 | 40 | 40 | 10 | 15 | 10 | |||||
| FTZN1E49 | 120 | 40 | 40 | 1 | 15 | 10 | |||||
| FTZN1E50 | 120 | 40 | 40 | 2 | 15 | 10 | |||||
| FTZN1E51 | 120 | 40 | 40 | 3 | 15 | 10 | |||||
| FTZN1E52 | 120 | 40 | 40 | 4 | 15 | 10 | |||||
| FTZN1E53 | 120 | 40 | 40 | 5 | 15 | 10 | |||||
| FTZN1E54 | 120 | 40 | 40 | 10 | 15 | 10 | |||||
| Formulation Code | Zero Order | First Order | Higuchi Order | Hixon Crowell | Korsmeyer | Release Mechanism | |
|---|---|---|---|---|---|---|---|
| R21 | R22 | R23 | R24 | R25 | N | ||
| FTZN1 | 0.923 | 0.923 | 0.956 | 0.978 | 0.967 | 0.623 | Non Fickian |
| FTZN2 | 0.954 | 0.934 | 0.923 | 0.987 | 0.945 | 0.689 | Non Fickian |
| FTZN3 | 0.987 | 0.967 | 0.989 | 0.967 | 0.962 | 0.634 | Non Fickian |
| FTZN4 | 0.934 | 0.923 | 0.934 | 0.934 | 0.934 | 0.667 | Non Fickian |
| FTZN5 | 0.953 | 0.967 | 0.967 | 0.934 | 0.954 | 0.701 | Non Fickian |
| FTZN6 | 0.934 | 0.945 | 0.961 | 0.956 | 0.956 | 0.698 | Non Fickian |
| FTZN7 | 0.964 | 0.945 | 0.923 | 0.967 | 0.978 | 0.656 | Non Fickian |
| FTZN8 | 0.967 | 0.923 | 0.912 | 0.989 | 0.932 | 0.756 | Non Fickian |
| FTZN9 | 0.988 | 0.998 | 0.993 | 0.992 | 0.934 | 0.907 | Zero Order |
| FTZN10 | 0.976 | 0.981 | 0.977 | 0.987 | 0.985 | 0.898 | Zero Order |
| FTZN11 | 0.945 | 0.983 | 0.923 | 0.934 | 0.954 | 0.864 | Non Fickian |
| FTZN12 | 0.934 | 0.967 | 0.958 | 0.923 | 0.924 | 0.783 | Non Fickian |
| FTZN13 | 0.934 | 0.945 | 0.961 | 0.956 | 0.956 | 0.698 | Non Fickian |
| FTZN14 | 0.964 | 0.945 | 0.923 | 0.967 | 0.978 | 0.656 | Non Fickian |
| FTZN15 | 0.967 | 0.923 | 0.912 | 0.989 | 0.932 | 0.756 | Non Fickian |
| FTZN16 | 0.988 | 0.998 | 0.993 | 0.992 | 0.934 | 0.907 | Zero Order |
| FTZN17 | 0.976 | 0.981 | 0.977 | 0.987 | 0.985 | 0.898 | Zero Order |
| FTZN18 | 0.945 | 0.983 | 0.923 | 0.934 | 0.954 | 0.864 | Non Fickian |
| FTZNE19 | 0.934 | 0.967 | 0.958 | 0.923 | 0.924 | 0.783 | Non Fickian |
| FTZNE20 | 0.923 | 0.923 | 0.956 | 0.978 | 0.967 | 0.623 | Non Fickian |
| FTZNE21 | 0.954 | 0.934 | 0.923 | 0.987 | 0.945 | 0.689 | Non Fickian |
| FTZNE22 | 0.987 | 0.967 | 0.989 | 0.967 | 0.962 | 0.634 | Non Fickian |
| FTZNE23 | 0.934 | 0.923 | 0.934 | 0.934 | 0.934 | 0.667 | Non Fickian |
| FTZNE24 | 0.953 | 0.967 | 0.967 | 0.934 | 0.954 | 0.701 | Non Fickian |
| FTZNE25 | 0.934 | 0.945 | 0.961 | 0.956 | 0.956 | 0.698 | Non Fickian |
| FTZNE26 | 0.964 | 0.945 | 0.923 | 0.967 | 0.978 | 0.656 | Non Fickian |
| FTZNE27 | 0.967 | 0.923 | 0.912 | 0.989 | 0.932 | 0.756 | Non Fickian |
| FTZNE28 | 0.923 | 0.923 | 0.956 | 0.978 | 0.967 | 0.623 | Non Fickian |
| FTZNE29 | 0.954 | 0.934 | 0.923 | 0.987 | 0.945 | 0.689 | Non Fickian |
| FTZNE30 | 0.987 | 0.967 | 0.989 | 0.967 | 0.962 | 0.634 | Non Fickian |
| FTZNE31 | 0.934 | 0.923 | 0.934 | 0.934 | 0.934 | 0.667 | Non Fickian |
| FTZNE32 | 0.953 | 0.967 | 0.967 | 0.934 | 0.954 | 0.701 | Non Fickian |
| FTZNE33 | 0.934 | 0.945 | 0.961 | 0.956 | 0.956 | 0.698 | Non Fickian |
| FTZNE34 | 0.964 | 0.945 | 0.923 | 0.967 | 0.978 | 0.656 | Non Fickian |
| FTZNE35 | 0.967 | 0.923 | 0.912 | 0.989 | 0.932 | 0.756 | Non Fickian |
| FTZNE36 | 0.988 | 0.998 | 0.993 | 0.992 | 0.934 | 0.907 | Zero Order |
| FTZNE37 | 0.976 | 0.981 | 0.977 | 0.987 | 0.985 | 0.898 | Zero Order |
| FTZNE38 | 0.945 | 0.983 | 0.923 | 0.934 | 0.954 | 0.864 | Non Fickian |
| FTZNE39 | 0.934 | 0.967 | 0.958 | 0.923 | 0.924 | 0.783 | Non Fickian |
| FTZNE40 | 0.934 | 0.945 | 0.961 | 0.956 | 0.956 | 0.698 | Non Fickian |
| FTZNE41 | 0.964 | 0.945 | 0.923 | 0.967 | 0.978 | 0.656 | Non Fickian |
| FTZNE42 | 0.967 | 0.923 | 0.912 | 0.989 | 0.932 | 0.756 | Non Fickian |
| FTZNE43 | 0.988 | 0.998 | 0.993 | 0.992 | 0.934 | 0.907 | Zero Order |
| FTZNE44 | 0.976 | 0.981 | 0.977 | 0.987 | 0.985 | 0.898 | Zero Order |
| FTZNE45 | 0.945 | 0.983 | 0.923 | 0.934 | 0.954 | 0.864 | Non Fickian |
| FTZNE46 | 0.934 | 0.967 | 0.958 | 0.923 | 0.924 | 0.783 | Non Fickian |
| FTZNE47 | 0.923 | 0.923 | 0.956 | 0.978 | 0.967 | 0.623 | Non Fickian |
| FTZNE48 | 0.954 | 0.934 | 0.923 | 0.987 | 0.945 | 0.689 | Non Fickian |
| FTZNE49 | 0.934 | 0.945 | 0.961 | 0.956 | 0.956 | 0.698 | Non Fickian |
| FTZNE50 | 0.964 | 0.945 | 0.923 | 0.967 | 0.978 | 0.656 | Non Fickian |
| FTZNE51 | 0.967 | 0.923 | 0.912 | 0.989 | 0.932 | 0.756 | Non Fickian |
| FTZNE52 | 0.988 | 0.998 | 0.993 | 0.992 | 0.934 | 0.907 | Zero Order |
| FTZNE53 | 0.976 | 0.981 | 0.977 | 0.987 | 0.985 | 0.898 | Zero Order |
| FTZNE54 | 0.945 | 0.983 | 0.923 | 0.934 | 0.954 | 0.864 | Non Fickian |
| Formulation Code | Weight (mg) | Drug Contents (%) | Thickness (mm) | Folding Endurance (times) | % Moisture Absorbance | % Moisture Loss | Hardness | Flatness |
|---|---|---|---|---|---|---|---|---|
| FTZN1 | 220.23 ± 0.006 | 98.12 ± 0.034 | 0.20 ± 0.002 | 180 | 8.32 ± 1.3 | 7.3 ± 1.6 | 213 ± 1.3 | 100 |
| FTZN2 | 225.67 ± 0.003 | 99.34 ± 0.034 | 0.21 ± 0.003 | 188 | 8.34 ± 1.6 | 7.5 ± 2.1 | 227 ± 1.8 | 99.98 |
| FTZN3 | 228.12 ± 0.005 | 98.87 ± 0.023 | 0.22 ± 0.005 | 198 | 8.7 ± 1.3 | 8.2 ± 2.4 | 233 ± 2.1 | 99.96 |
| FTZN4 | 232.23 ± 0.014 | 99.64 ± 0.031 | 0.21 ± 0.007 | 206 | 8.1 ± 1.9 | 7.8 ± 1.9 | 240 ± 1.9 | 99.98 |
| FTZN5 | 235.89 ± 0.018 | 98.37 ± 0.121 | 0.23 ± 0.004 | 216 | 9.2 ± 1.4 | 8.8 ± 1.7 | 237 ± 1.6 | 100 |
| FTZN6 | 230.34 ± 0.018 | 97.25 ± 0.023 | 0.24 ± 0.007 | 205 | 9.4 ± 1.7 | 8.6 ± 2.4 | 242 ± 1.8 | 99.88 |
| FTZN7 | 225.21 ± 0.006 | 99.11 ± 0.034 | 0.21 ± 0.002 | 181 | 8.32 ± 1.3 | 7.5 ± 1.6 | 215 ± 1.3 | 99.78 |
| FTZN8 | 228.45 ± 0.003 | 98.56 ± 0.034 | 0.23 ± 0.003 | 183 | 8.35 ± 1.6 | 7.3 ± 2.1 | 223 ± 1.8 | 99.92 |
| FTZN9 | 230.14 ± 0.005 | 99.34 ± 0.023 | 0.21 ± 0.005 | 194 | 8.24 ± 1.3 | 8.3 ± 2.4 | 235 ± 2.1 | 99.90 |
| FTZN10 | 233.25 ± 0.014 | 98.23 ± 0.031 | 0.22 ± 0.007 | 202 | 8.81 ± 1.9 | 7.6 ± 1.9 | 236 ± 1.9 | 99.85 |
| FTZN11 | 238.56 ± 0.018 | 99.45 ± 0.121 | 0.22 ± 0.004 | 212 | 9.34 ± 1.4 | 8.7 ± 1.7 | 236 ± 1.6 | 99.56 |
| FTZN12 | 232.45 ± 0.018 | 99.12 ± 0.023 | 0.24 ± 0.007 | 208 | 9.62 ± 1.7 | 8.8 ± 2.4 | 240 ± 1.8 | 99.92 |
| FTZN13 | 38.23 ± 0.004 | 98.12 ± 0.034 | 0.21 ± 0.002 | 175 | 10.32 ± 1.3 | 7.3 ± 1.6 | 214 ± 1.3 | 100 |
| FTZN14 | 39.67 ± 0.005 | 99.34 ± 0.034 | 0.23 ± 0.003 | 190 | 9.34 ± 1.6 | 8.1 ± 2.1 | 221 ± 1.8 | 100 |
| FTZN15 | 39.12 ± 0.008 | 98.97 ± 0.023 | 0.24 ± 0.005 | 197 | 7.9 ± 1.3 | 7.9 ± 2.4 | 231 ± 2.1 | 99.98 |
| FTZN16 | 37.23 ± 0.012 | 97.34 ± 0.031 | 0.23 ± 0.007 | 211 | 8.1 ± 1.9 | 8.2 ± 1.9 | 234 ± 1.9 | 100 |
| FTZN17 | 36.89 ± 0.017 | 96.67 ± 0.121 | 0.25 ± 0.004 | 201 | 9.2 ± 1.4 | 7.8 ± 1.7 | 237 ± 1.6 | 99.98 |
| FTZN18 | 38.34 ± 0.019 | 97.45 ± 0.023 | 0.24 ± 0.007 | 199 | 8.4 ± 1.7 | 7.3 ± 2.4 | 239 ± 1.8 | 99.99 |
| FTZNE19 | 37.23 ± 0.034 | 98.35 ± 0.004 | 0.26 ± 0.008 | 191 | 10.4 ± 1.2 | 6.9 ± 1.9 | 248 ± 2.1 | 99.98 |
| FTZNE20 | 38.45 ± 0.023 | 99.21 ± 0.043 | 0.27 ± 0.005 | 197 | 8.9 ± 1.5 | 6.5 ± 1.5 | 251 ± 2.8 | 99.98 |
| FTZNE21 | 37.17 ± 0.021 | 97.32 ± 0.021 | 0.24 ± 0.004 | 208 | 9.2 ± 1.3 | 7.3 ± 2.1 | 237 ± 1.9 | 100 |
| FTZNE22 | 38.56 ± 0.045 | 98.36 ± 0.004 | 0.26 ± 0.005 | 212 | 8.9 ± 1.5 | 7.8 ± 1.8 | 230 ± 1.7 | 99.99 |
| FTZNE23 | 39.21 ± 0.005 | 98.34 ± 0.023 | 0.23 ± 0.008 | 231 | 9.4 ± 1.8 | 6.2 ± 1.9 | 238 ± 2.3 | 99.97 |
| FTZNE24 | 38.19 ± 0.034 | 97.54 ± 0.012 | 0.22 ± 0.003 | 215 | 8.1 ± 1.3 | 7.3 ± 1.6 | 231 ± 1.9 | 100 |
| FTZNE25 | 39.23 ± 0.006 | 99.12 ± 0.034 | 0.23 ± 0.002 | 178 | 10.32 ± 1.3 | 7.3 ± 1.6 | 212 ± 1.3 | 99.99 |
| FTZNE26 | 38.67 ± 0.003 | 98.34 ± 0.034 | 0.22 ± 0.003 | 198 | 8.34 ± 1.6 | 8.1 ± 2.1 | 225 ± 1.8 | 100 |
| FTZNE27 | 38.12 ± 0.005 | 99.97 ± 0.023 | 0.25 ± 0.005 | 195 | 9.9 ± 1.3 | 7.9 ± 2.4 | 237 ± 2.1 | 100 |
| FTZNE28 | 39.23 ± 0.014 | 98.34 ± 0.031 | 0.22 ± 0.007 | 212 | 7.1 ± 1.9 | 8.2 ± 1.9 | 239 ± 1.9 | 100 |
| FTZNE29 | 37.89 ± 0.018 | 97.67 ± 0.121 | 0.24 ± 0.004 | 221 | 8.2 ± 1.4 | 7.8 ± 1.7 | 234 ± 1.6 | 100 |
| FTZNE30 | 38.34 ± 0.018 | 96.45 ± 0.023 | 0.25 ± 0.007 | 197 | 9.4 ± 1.7 | 7.3 ± 2.4 | 241 ± 1.8 | 99.99 |
| FTZNE31 | 39.23 ± 0.033 | 99.35 ± 0.004 | 0.27 ± 0.008 | 196 | 10.4 ± 1.2 | 6.9 ± 1.9 | 245 ± 2.1 | 99.97 |
| FTZNE32 | 39.45 ± 0.021 | 98.21 ± 0.043 | 0.26 ± 0.005 | 199 | 9.9 ± 1.5 | 6.5 ± 1.5 | 252 ± 2.8 | 100 |
| FTZNE33 | 35.17 ± 0.029 | 98.32 ± 0.021 | 0.25 ± 0.004 | 205 | 8.2 ± 1.3 | 7.3 ± 2.1 | 243 ± 1.9 | 99.98 |
| FTZNE34 | 37.56 ± 0.047 | 99.36 ± 0.004 | 0.28 ± 0.005 | 211 | 9.9 ± 1.5 | 7.8 ± 1.8 | 236 ± 1.7 | 100 |
| FTZNE35 | 38.21 ± 0.008 | 97.34 ± 0.023 | 0.23 ± 0.008 | 211 | 8.4 ± 1.8 | 6.2 ± 1.9 | 234 ± 2.3 | 99.97 |
| FTZNE36 | 39.19 ± 0.035 | 98.54 ± 0.012 | 0.24 ± 0.003 | 216 | 9.1 ± 1.3 | 7.3 ± 1.6 | 238 ± 1.9 | 100 |
| FTZNE37 | 38.56 ± 0.045 | 98.36 ± 0.004 | 0.26 ± 0.005 | 212 | 8.9 ± 1.5 | 7.8 ± 1.8 | 230 ± 1.7 | 99.99 |
| FTZNE38 | 39.21 ± 0.005 | 98.34 ± 0.023 | 0.23 ± 0.008 | 231 | 9.4 ± 1.8 | 6.2 ± 1.9 | 238 ± 2.3 | 99.97 |
| FTZNE39 | 38.19 ± 0.034 | 97.54 ± 0.012 | 0.22 ± 0.003 | 215 | 8.1 ± 1.3 | 7.3 ± 1.6 | 231 ± 1.9 | 100 |
| FTZNE40 | 39.23 ± 0.006 | 99.12 ± 0.034 | 0.23 ± 0.002 | 178 | 10.32 ± 1.3 | 7.3 ± 1.6 | 212 ± 1.3 | 99.99 |
| FTZNE41 | 38.67 ± 0.003 | 98.34 ± 0.034 | 0.22 ± 0.003 | 198 | 8.34 ± 1.6 | 8.1 ± 2.1 | 225 ± 1.8 | 100 |
| FTZNE42 | 38.12 ± 0.005 | 99.97 ± 0.023 | 0.25 ± 0.005 | 195 | 9.9 ± 1.3 | 7.9 ± 2.4 | 237 ± 2.1 | 100 |
| FTZNE43 | 39.23 ± 0.014 | 98.34 ± 0.031 | 0.22 ± 0.007 | 212 | 7.1 ± 1.9 | 8.2 ± 1.9 | 239 ± 1.9 | 100 |
| FTZNE44 | 38.45 ± 0.023 | 99.21 ± 0.043 | 0.27 ± 0.005 | 197 | 8.9 ± 1.5 | 6.5 ± 1.5 | 251 ± 2.8 | 99.98 |
| FTZNE45 | 37.17 ± 0.021 | 97.32 ± 0.021 | 0.24 ± 0.004 | 208 | 9.2 ± 1.3 | 7.3 ± 2.1 | 237 ± 1.9 | 100 |
| FTZNE46 | 38.56 ± 0.045 | 98.36 ± 0.004 | 0.26 ± 0.005 | 212 | 8.9 ± 1.5 | 7.8 ± 1.8 | 230 ± 1.7 | 99.99 |
| FTZNE47 | 39.21 ± 0.005 | 98.34 ± 0.023 | 0.23 ± 0.008 | 231 | 9.4 ± 1.8 | 6.2 ± 1.9 | 238 ± 2.3 | 99.97 |
| FTZNE48 | 38.19 ± 0.034 | 97.54 ± 0.012 | 0.22 ± 0.003 | 215 | 8.1 ± 1.3 | 7.3 ± 1.6 | 231 ± 1.9 | 100 |
| FTZNE49 | 39.23 ± 0.006 | 99.12 ± 0.034 | 0.23 ± 0.002 | 178 | 10.32 ± 1.3 | 7.3 ± 1.6 | 212 ± 1.3 | 99.99 |
| FTZNE50 | 38.67 ± 0.003 | 98.34 ± 0.034 | 0.22 ± 0.003 | 198 | 8.34 ± 1.6 | 8.1 ± 2.1 | 225 ± 1.8 | 100 |
| FTZNE51 | 38.12 ± 0.005 | 99.97 ± 0.023 | 0.25 ± 0.005 | 195 | 9.9 ± 1.3 | 7.9 ± 2.4 | 237 ± 2.1 | 100 |
| FTZNE52 | 39.23 ± 0.014 | 98.34 ± 0.031 | 0.22 ± 0.007 | 212 | 7.1 ± 1.9 | 8.2 ± 1.9 | 239 ± 1.9 | 100 |
| FTZNE53 | 37.89 ± 0.018 | 97.67 ± 0.121 | 0.24 ± 0.004 | 221 | 8.2 ± 1.4 | 7.8 ± 1.7 | 234 ± 1.6 | 100 |
| FTZNE54 | 38.34 ± 0.018 | 96.45 ± 0.023 | 0.25 ± 0.007 | 197 | 9.4 ± 1.7 | 7.3 ± 2.4 | 241 ± 1.8 | 99.99 |
| Evaluation Parameter | F. Code | 1st Month | 2nd Month | 3rd Month | 5th Month | 6th Month |
|---|---|---|---|---|---|---|
| Drug contents | FTZNE6 | 99.45 ± 1.78 | 99.26 ± 1.22 | 99.23 ± 1.79 | 98.67 ± 1.09 | 98.98 ± 1.92 |
| FTZNE12 | 99.78 ± 1.66 | 98.89 ± 1.35 | 98.56 ± 1.88 | 97.78 ± 1.45 | 97.23 ± 188 | |
| 99.23 ± 1.34 | 98.78 ± 1.22 | 97.78 ± 1.99 | 98.48 ± 1.33 | 98.12 ± 1.66 | ||
| FTZNE24 | 99.86 ± 1.22 | 99.45 ± 1.11 | 99.67 ± 1.77 | 99.23 ± 1.73 | 99.10 ± 1.33 | |
| FTZNE30 | 99.78 ± 1.66 | 98.89 ± 1.10 | 98.56 ± 1.56 | 97.78 ± 1.65 | 97.23 ± 1.35 | |
| FTZNE36 | 99.86 ± 1.22 | 99.45 ± 1.54 | 99.67 ± 1.45 | 99.23 ± 1.11 | 99.10 ± 1.78 | |
| Appearance | FTZNE6 | No change | No change | No change | No change | No change |
| FTZNE12 | No change | No change | No change | No change | No change | |
| FTZNE18 | No change | No change | No change | No change | No change | |
| FTZNE24 | No change | No change | No change | No change | No change | |
| FTZNE30 | No change | No change | No change | No change | No change | |
| FTZNE36 | No change | No change | No change | No change | No change |
| Formulation Code | Visual Observation | |
|---|---|---|
| Erythema | Edema | |
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Adhesive tape | 0.94 ± 0.63 | 1.03 ± 0.76 |
| Blank Patch | 1.18 ± 0.67 | 1.05 ± 0.31 |
| FTZNE6 | 0.98 ± 0.45 | 0.85 ± 0.45 |
| FTZNE12 | 0.90 ± 0.34 | 1.04 ± 0.23 |
| FTZNE18 | 0.86 ± 0.43 | 1.05 ± 0.23 |
| FTZNE24 | 0.96 ± 0.23 | 1.06 ± 0.52 |
| FTZNE30 | 1.02 ± 0.26 | 1.08 ± 0.56 |
| FTZNE36 | 0.93 ± 0.34 | 1.03 ± 0.45 |
| FTZNE42 | 0.98 ± 0.56 | 1.07 ± 0.53 |
| FTZNE48 | 1.03 ± 0.34 | 1.09 ± 0.75 |
| FTZNE54 | 0.95 ± 0.43 | 1.04 ± 0.32 |
| Formaline | 3.05 ± 0.65 | 3.4 ± 0.37 |
| Formulation Code | Flux (µg/cm2·h) ± S.D. | Kp (cm/h) ± S.D. | ER | Tlag(h) ± S.D. |
|---|---|---|---|---|
| Control | 21.53 ± 1.72 | 0.689 ± 0.002 | 1.00 | 3.87 ± 0.006 |
| FTZN1 | 21.21 ± 2.12 | 0.310 ± 0.006 | 1.34 | 3.08 ± 0.004 |
| FTZN2 | 35.23 ± 2.72 | 0.611 ± 0.003 | 3.32 | 3.35 ± 0.003 |
| FTZN3 | 60.34 ± 1.93 | 0.987 ± 0.023 * | 7.19 | 2.75 ± 0.006 |
| FTZN4 | 87.23 ± 3.54 | 1.113 ± 0.034 * | 12.48 | 2.24 ± 0.008 |
| FTZN5 | 112.34 ± 3.22 | 3.112 ± 0.052 * | 15.85 | 2.13 ± 0.011 |
| FTZN6 | 139.45 ± 4.23 | 4.431 ± 0.004 * | 17.84 | 1.43 ± 0.012 |
| FTZN7 | 31.23 ± 2.12 | 0.902 ± 0.006 | 1.23 | 3.07 ± 0.004 |
| FTZN8 | 48.67 ± 2.72 | 1.564 ± 0.003 | 2.39 | 3.45 ± 0.003 |
| FTZN9 | 86.45 ± 1.93 | 2.962 ± 0.023 * | 4.24 | 2.87 ± 0.006 |
| FTZN10 | 116.23 ± 3.54 | 4.112 ± 0.034 * | 5.58 | 2.34 ± 0.008 |
| FTZN11 | 241.67 ± 4.23 | 5.564 ± 0.004 * | 7.94 | 1.56 ± 0.012 |
| FTZN12 | 181.34 ± 3.22 | 5.103 ± 0.052 * | 6.95 | 2.03 ± 0.011 |
| FTZN13 | 29.23 ± 2.12 | 0.902 ± 0.006 | 1.21 | 3.12 ± 0.004 |
| FTZN14 | 45.67 ± 2.72 | 1.573 ± 0.003 | 2.34 | 3.67 ± 0.003 |
| FTZN15 | 83.45 ± 1.93 | 2.987 ± 0.023 * | 4.21 | 2.98 ± 0.006 |
| FTZN16 | 115.23 ± 3.54 | 4.103 ± 0.034 * | 5.63 | 2.56 ± 0.008 |
| FTZN17 | 179.34 ± 3.22 | 5.112 ± 0.052 * | 6.98 | 2.13 ± 0.011 |
| FTZN18 | 239.67 ± 4.23 | 5.621 ± 0.004 * | 7.88 | 1.87 ± 0.012 |
| FTZNE19 | 31.23 ± 2.23 | 1.123 ± 0.007 | 1.32 | 2.97 ± 0.004 |
| FTZNE20 | 61.87 ± 2.34 | 1.987 ± 0.005 | 2.28 | 1.67 ± 0.003 |
| FTZNE21 | 103.45 ± 1.56 | 3.123 ± 0.054 * | 4.34 | 1.98 ± 0.006 |
| FTZNE22 | 134.23 ± 3.23 | 4.321 ± 0.023 * | 5.98 | 2.21 ± 0.008 |
| FTZNE23 | 211.67 ± 4.12 | 6.342 ± 0.007 * | 8.98 | 1.23 ± 0.012 |
| FTZNE24 | 205.34 ± 3.13 | 6.112 ± 0.072 * | 8.34 | 2.23 ± 0.011 |
| FTZNE25 | 32.67 ± 2.12 | 0.934 ± 0.006 | 1.45 | 3.07 ± 0.004 |
| FTZNE26 | 49.27 ± 2.72 | 1.632 ± 0.003 | 2.78 | 3.45 ± 0.003 |
| FTZNE27 | 87.78 ± 1.93 | 2.998 ± 0.023 * | 4.89 | 2.87 ± 0.006 |
| FTZNE28 | 117.45 ± 3.54 | 4.221 ± 0.034 * | 5.39 | 2.34 ± 0.008 |
| FTZNE29 | 182.78 ± 3.22 | 5.112 ± 0.052 * | 7.12 | 2.03 ± 0.011 |
| FTZNE30 | 242.89 ± 4.23 | 5.473 ± 0.004 * | 8.22 | 1.56 ± 0.012 |
| FTZNE31 | 29.23 ± 2.12 | 0.902 ± 0.006 | 1.21 | 3.12 ± 0.004 |
| FTZNE32 | 45.67 ± 2.72 | 1.573 ± 0.003 | 2.34 | 3.67 ± 0.003 |
| FTZNE33 | 83.45 ± 1.93 | 2.987 ± 0.023 * | 4.21 | 2.98 ± 0.006 |
| FTZNE34 | 115.23 ± 3.54 | 4.103 ± 0.034 * | 5.63 | 2.56 ± 0.008 |
| FTZNE35 | 239.67 ± 4.23 | 5.621 ± 0.004 * | 7.88 | 1.87 ± 0.012 |
| FTZNE36 | 179.34 ± 3.22 | 5.112 ± 0.052 * | 6.98 | 2.13 ± 0.011 |
| FTZNE37 | 21.21 ± 2.12 | 0.310 ± 0.006 | 1.34 | 3.08 ± 0.004 |
| FTZNE38 | 35.23 ± 2.72 | 0.611 ± 0.003 | 3.32 | 3.35 ± 0.003 |
| FTZNE39 | 60.34 ± 1.93 | 0.987 ± 0.023 * | 7.19 | 2.75 ± 0.006 |
| FTZNE40 | 87.23 ± 3.54 | 1.113 ± 0.034 * | 12.48 | 2.24 ± 0.008 |
| FTZNE41 | 112.34 ± 3.22 | 3.112 ± 0.052 * | 15.85 | 2.13 ± 0.011 |
| FTZNE42 | 139.45 ± 4.23 | 4.431 ± 0.004 * | 17.84 | 1.43 ± 0.012 |
| FTZNE43 | 31.23 ± 2.12 | 0.902 ± 0.006 | 1.23 | 3.07 ± 0.004 |
| FTZNE44 | 48.67 ± 2.72 | 1.564 ± 0.003 | 2.39 | 3.45 ± 0.003 |
| FTZNE45 | 86.45 ± 1.93 | 2.962 ± 0.023 * | 4.24 | 2.87 ± 0.006 |
| FTZNE46 | 116.23 ± 3.54 | 4.112 ± 0.034 * | 5.58 | 2.34 ± 0.008 |
| FTZNE47 | 241.67 ± 4.23 | 5.564 ± 0.004 * | 7.94 | 1.56 ± 0.012 |
| FTZNE48 | 181.34 ± 3.22 | 5.103 ± 0.052 * | 6.95 | 2.03 ± 0.011 |
| FTZNE49 | 31.23 ± 2.23 | 1.123 ± 0.007 | 1.32 | 2.97 ± 0.004 |
| FTZNE50 | 61.87 ± 2.34 | 1.987 ± 0.005 | 2.28 | 1.67 ± 0.003 |
| FTZNE51 | 103.45 ± 1.56 | 3.123 ± 0.054 * | 4.34 | 1.98 ± 0.006 |
| FTZNE52 | 134.23 ± 3.23 | 4.321 ± 0.023 * | 5.98 | 2.21 ± 0.008 |
| FTZNE53 | 205.34 ± 3.13 | 6.112 ± 0.072 * | 8.34 | 2.23 ± 0.011 |
| FTZN E54 | 211.67 ± 4.12 | 6.342 ± 0.007 * | 8.98 | 1.23 ± 0.012 |
| Formulation Code | Cmax (Hours) | Tmax | AUC0-α (µg/mL/h) | Relative Bioavailability | Half-Life t1/2 | MRT0-α (Hours) | Cl (mL/min) |
|---|---|---|---|---|---|---|---|
| Oral Marketed Tablets | 27.56 ± 2.12 | 1.0 | 92.06 ± 3.23 | - | 2.98 ± 0.37 | 3.81 ± 0.34 | 0.71 ± 0.29 |
| Control patch | 8.92 ± 2.67 | 8.0 | 182 ± 1.78 | 1.85 | 8.81 ± 0.18 | 10.89 ± 1.67 | 0.06 ± 0.02 |
| FTZNE23 | 15.78 ± 2.45 * | 8.0 | 280.36 ± 1.45 | 3.01 * | 9.98 ± 0.52 * | 12.96 ± 1.56 | 0.12 ± 0.01 |
| FTZNE36 | 25.14 ± 1.23 * | 8.0 | 360.45 ± 2.34 | 3.92 * | 10.93 ± 0.68 * | 15.13 ± 1.67 | 0.04 ± 0.02 |
| FTZNE54 | 36.21 ± 2.31 * | 8.0 | 530.67 ± 1.45 | 4.23 * | 10.73 ± 0.45 * | 16.34 ± 1.45 | 0.05 ± 0.04 |
References
- Kumar, A.; Pullankandam, N.; Prabhu, S.L.; Gopal, V. Transdermal drug delivery system: An overview. Int. J. Pharm. Sci. Rev. Res. 2010, 3, 49–54. [Google Scholar]
- Rao, P.R.; Ramakrishna, S.; Diwan, P.V. Drug Release Kinetics from Polymeric Films Containing Propranolol Hydrochloride for Transdermal Use. Pharm. Dev. Tech. 2000, 5, 465–472. [Google Scholar] [CrossRef]
- Chen, J.; Gao, Y. Strategies for meloxicam delivery to and across the skin: A review. Drug Deliv. 2016, 23, 3146–3156. [Google Scholar] [CrossRef] [Green Version]
- Latif, M.S.; Azad, A.K.; Nawaz, A.; Rashid, S.A.; Rahman, M.; Al Omar, S.Y.; Abdel-Daim, M.M. Ethyl Cellulose and Hydroxypropyl Methyl Cellulose Blended Methotrexate-Loaded Transdermal Patches: In Vitro and Ex Vivo. Polymers 2021, 13, 3455. [Google Scholar] [CrossRef]
- Khan, T.A.; Azad, A.K.; Fuloria, S.; Nawaz, A.; Subramaniyan, V.; Akhlaq, M.; Fuloria, N.K. Chitosan-Coated 5-Fluorouracil Incorporated Emulsions as Transdermal Drug Delivery Matrices. Polymers 2021, 13, 3345. [Google Scholar] [CrossRef]
- Aggarwal, G.; Dhawan, S.; Hari Kumar, S.L. Natural oils as skin permeation enhancers for transdermal delivery of olanzapine: In vitro and in vivo evaluation. Curr. Drug Deliv. 2012, 9, 172–181. [Google Scholar] [CrossRef]
- Marwah, H.; Garg, T.; Goyal, A.K.; Rath, G. Permeation enhancer strategies in transdermal drug delivery. Drug Deliv. 2016, 23, 564–578. [Google Scholar] [CrossRef]
- Swain, K.; Pattnaik, S.; Sahu, S.C.; Patnaik, K.K.; Mallick, S. Drug in adhesive type transdermal matrix systems of ondansetron hydrochloride: Optimization of permeation pattern using response surface methodology. J. Drug Target. 2010, 18, 106–114. [Google Scholar] [CrossRef]
- Mohsen, A.M.; Salama, A.; Kassem, A.A. Development of acetazolamide loaded bilosomes for improved ocular delivery: Preparation, characterization and in vivo evaluation. Drug Deliv. Sci. Technol. 2020, 59, 101910. [Google Scholar] [CrossRef]
- Setty, C.M.; Jawarkar, Y.; Pathan, I.B. Effect of essential oils as permeation enhancers on percutaneous penetration of furosemide through human cadaver skin. Acta Pharm. Sci. 2010, 52, 159–168. [Google Scholar]
- Jain, R.; Aqil, M.; Ahad, A.; Ali, A.; Khar, R.K. Basil oil is a promising skin penetration enhancer for transdermal delivery of labetolol hydrochloride. Drug Dev. Ind. Pharm. 2008, 34, 384–389. [Google Scholar] [CrossRef] [PubMed]
- El-Waseif, M.A.; Hashem, H.A.; Abd El-Dayem, H.H. Using flaxseed oil to prepare therapeutical fat spreads. Ann. Agric. Sci. 2013, 58, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, G.; Dhawan, S.; Hari Kumar, S.L. Formulation, in vitro and in vivo evaluation of transdermal patches containing risperidone. Drug Dev. Ind. Pharm. 2013, 39, 39–50. [Google Scholar] [CrossRef]
- Galipoğlu, M.; Erdal, M.S.; Güngör, S. Biopolymer-based transdermal films of donepezil as an alternative delivery approach in Alzheimer’s disease treatment. AAPS PharmSciTech 2015, 16, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Azad, A.K.; Doolaanea, A.A.; Al-Mahmood, S.M.A.; Kennedy, J.F.; Chatterjee, B.; Bera, H. Electro-hydrodynamic assisted synthesis of lecithin-stabilized peppermint oil-loaded alginate microbeads for intestinal drug delivery. Int. J. Biol. Macromol. 2021, 185, 861–875. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.K.; Al-Mahmood, S.M.A.; Chatterjee, B.; Sulaiman, W.M.A.; Elsayed, T.M.; Doolaanea, A.A. Encapsulation of black seed oil in alginate beads as a ph-sensitive carrier for intestine-targeted drug delivery: In vitro, in vivo and ex vivo study. Pharmaceutics 2020, 12, 219. [Google Scholar] [CrossRef] [Green Version]
- Munada, A.S.; Avari, J.G. Evaluation of gum copal as rate controlling membrane for transdermal application: Effect of plasticizers. Acta Pharm. Sci. 2010, 52, 31–38. [Google Scholar]
- De, P.; Damodharan, N.; Mallick, S.; Mukherjee, B. Development and evaluation of nefopam transdermal matrix patch system in human volunteers. PDA J. Pharm. Sci. Technol. 2009, 63, 537–546. [Google Scholar]
- Chandak, A.R.; Prasad Verma, P.R. Eudragit-based transdermal delivery system of pentazocine: Physico-chemical, in vitro and in vivo evaluations. Pharm. Dev. Technol. 2010, 15, 296–304. [Google Scholar] [CrossRef]
- Mittal, A.; Parmar, S.; Singh, B. In vitro and in vivo assessment of matrix type transdermal therapeutic system of Labetolol hydrochloride. Curr. Drug Deliv. 2009, 6, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Walash, M.I.; Belal, F.F.; Eid, M.I.; Mohamed, S.A.E.A. Spectrophotometric determination of tizanidine and orphenadrine via ion pair complex formation using eosin Y. Chem. Cent. J. 2011, 5, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, A.; Amaro, M.I.; Healy, A.M.; Cabral, L.M.; de Sousa, V.P. Development of a discriminative in vitro release test for rivastigmine transdermal patches using pharmacopeial apparatuses: USP 5 and USP 6. AAPS PharmSciTech 2017, 18, 2561–2569. [Google Scholar] [CrossRef]
- Ritger, R.L.; Peppas, N.S. A simple equation for disposition of solute release II: Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar]
- Korsmeyer, R.W.; Peppas, N.A. Effect of the Morphology of Hydrophilic Polymeric Matrices on the Diffusion and Release of Water-Soluble Drugs. J. Membr. Sci. 1981, 9, 211–227. [Google Scholar] [CrossRef]
- Higuchi, T. Mechanism of sustained action medication, theoretical analysis of rate of release of solid drugs dispersed in solid matrices. J. Pharm. Sci. 1963, 52, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Charoo, N.A.; Anwar, A.; Kohli, K.; Pillai, K.K.; Rahman, Z. Transdermal Delivery of Flurbiprofen: Permeation Enhancement, Design, Pharmacokinetic and Pharmacodynamic Studies in Albino Rats. Pharm. Dev. Technol. 2005, 10, 343–351. [Google Scholar] [CrossRef]
- Yener, G.; Under, M.; Gonullu, S.; Kilic, P.; Aslan, S.S.; Barla, A. Design of meloxicam and lornoxicam transdermal patchs: Preparation, physical characterization, ex vivo and in vivo studies. Chem. Pharm. Bull. 2010, 58, 1466–1473. [Google Scholar] [CrossRef] [Green Version]
- Yuk, S.H.; Lee, S.J. One-way membrane for transdermal drug delivery system. II. System optimization. Int. J. Pharm. 1991, 77, 231–237. [Google Scholar]
- Zaman, M.; Hanif, M.; Khan, N.U.H.; Mahmood, A.; Qaisar, M.N.; Ali, H. Development and validation of stability-indicating RP-HPLC method for the simultaneous determination of Tizanidine HCl and meloxicam in Rabbit’s plasma. Acta Chromatogr. 2019, 31, 173–178. [Google Scholar] [CrossRef]
- Moawad, F.A.; Ali, A.A.; Salem, H.F. Nanotransfersomes-loaded thermosensitive in situ gel as a rectal delivery system of tizanidine HCl: Preparation, in vitro and in vivo performance. Drug Deliv. 2017, 24, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pramanik, S.; Thakkar, H. Development of solid self-microemulsifying system of tizanidine hydrochloride for oral bioavailability enhancement: In vitro and in vivo evaluation. AAPS PharmSciTech 2020, 21, 182. [Google Scholar] [CrossRef]
- Khalil, R.M.; Abdelbary, A.; Arini, S.K.E.; Basha, M.; El-Hashemy, H.A.; Farouk, F. Development of tizanidine loaded aspasomes as transdermal delivery system: Ex-vivo and in-vivo evaluation. J. Liposome Res. 2021, 31, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Bera, H.; Abbasi, Y.F.; Gajbhiye, V.; Liew, K.F.; Kumar, P.; Tambe, P.; Azad, A.K.; Cun, D.; Yang, M. Carboxymethyl fenugreek galactomannan-g-poly (N-isopropylacrylamide-co-N,N’-methylene-bis-acrylamide)-clay based pH/temperature-responsive nanocomposites as drug-carriers. Mater. Sci. Eng. C 2020, 110, 110628. [Google Scholar] [CrossRef]
- Barkatiya, M.; Nema, R.K.; Bhatnagar, M. Designing and characterization of drug free patches for transdermal application. Int. J. Pharm. Sci. Drug Res. 2010, 2, 35–39. [Google Scholar]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Shahid, N.; Siddique, M.I.; Razzaq, Z.; Katas, H.; Waqas, M.K.; Rahman, K.U. Fabrication and characterization of matrix type transdermal patches loaded with tizanidine hydrochloride: Potential sustained release delivery system. Drug Dev. Ind. Pharm. 2018, 44, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Azim, A.A.; El-Ashmoony, M.; Swealem, A.M.; Shoukry, R.A. Transdermal films containing tizanidine: In vitro and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2014, 24, 92–99. [Google Scholar] [CrossRef]
- Kaul, N.; Dhaneshwar, S.R.; Agrawal, H.; Kakad, A.; Patil, B. Application of HPLC and HPTLC for the simultaneous determination of tizanidine and rofecoxib in pharmaceutical dosage form. J. Pharm. Biomed. Anal. 2005, 37, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Mahapatra, S.; Gupta, R.; Patra, B.; Tiwari, A.; Arora, P. A comparison between povidone-ethylcellulose and povidone-eudragit transdermal dexamethasone matrix patches based on in vitro skin permeation. Eur. J. Pharm. Biopharm. 2005, 59, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, M.; Fang, L. Biomaterials as novel penetration enhancers for transdermal and dermal drug delivery systems. Drug Deliv. 2013, 20, 199–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutalik, S.; Udapa, N. Glibenclamide transdermal patches: Physicochemical, pharmacodynamics and pharmacokinetics evaluation. J. Pharm. Sci. 2004, 93, 1577–1594. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Mukhterjee, B. Design, Development and invitro and in vivo evaluation of transdermal patches containing diclofenac diethylammonium salt. J. Pharm. Sci. 2002, 91, 2076–2089. [Google Scholar] [CrossRef] [PubMed]
- Jagdale, S.; Brahmane, S.; Chabukswar, A. Optimization of Microemulgel for Tizanidine Hydrochloride. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2020, 19, 158–179. [Google Scholar] [CrossRef]
- Pavithra, K.; Bhagawati, S.T.; Manjunath, K. Development and evaluation of tizanidine hydrochloride loaded solid lipid nanoparticles. Asian J. Pharm. Clin. Res. 2019, 12, 152–158. [Google Scholar] [CrossRef]
- Kittur, F.; Prashanth, K.H.; Sankar, K.U.; Tharanathan, R. Characterization of chitin, chitosan and their carboxymethyl derivatives by differential scanning calorimetry. Carbohydr. Polym. 2020, 49, 185–193. [Google Scholar] [CrossRef]
- Akram, R.; Ahmad, M.; Abrar, A.; Sarfraz, R.M.; Mahmood, A. Formulation design and development of matrix diffusion controlled transdermal drug delivery of glimepiride. Drug Des. Dev. Ther. 2018, 12, 349–364. [Google Scholar] [CrossRef] [Green Version]
- El-Gendy, N.A.; Sabry, N.A.; El-Attar, M.; Omar, E.; Mahmoud, M. Transdermal patch incorporating salbutamol sulphate: In vitro and clinical characterization. Drug Discov. Ther. 2008, 2, 219–228. [Google Scholar]
- Rao, P.R.; Rddy, M.N.; Ramakrishma, S.; Diwan, P.V. Comparative in vivo evaluation of propranolol hydrochloride after oral and transdermal administration in Rabbits. Eur. J. Pharm. Biopharm. 2003, 56, 81–85. [Google Scholar] [PubMed]
- Kolling, W.M. Handbook of pharmaceutical excipients. Am. J. Pharm. Educ. 2004, 68, BF1–BF2. [Google Scholar]
- Merkle, H.P.; Knoch, A.; Gienger, G. Release kinetics of polymeric laminates for transdermal drug delivery: Experimental evaluation and physical modeling. J. Control. Release 1985, 2, 99–110. [Google Scholar] [CrossRef]
- Satheeshababu, B.K.; Shivakumar, K.L. Synthesis of Conjugated Chitosan and its Effect on Drug Permeation from Transdermal Patches. Indian J. Pharm. Sci. 2013, 75, 162–170. [Google Scholar]
- Akhlaq, M.; Azad, A.K.; Ullah, I.; Nawaz, A.; Safdar, M.; Bhattacharya, T.; Nagaswarupa, H.P. Methotrexate-Loaded Gelatin and Polyvinyl Alcohol (Gel/PVA) Hydrogel as a pH-Sensitive Matrix. Polymers 2021, 13, 2300. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Jha, P.K. Recent Advances in Dissolution Testing and Their Use to Improve In Vitro–In Vivo Correlations in Oral Drug Formulations. J. Pharm. Innov. 2021, 1–16. [Google Scholar] [CrossRef]
- Hollenbeck, K.G.; Swarbrick, J.; Boylan, J.C. (Eds.) Encyclopedia of Pharmaceutical Technology; Dekker: New York, NY, USA, 1994; Volume 10, pp. 67–69. [Google Scholar]
- Blesa, R.; Ballard, C.; Orgogozo, J.; Lane, R.; Thomas, S.K. Caregiver preference for rivastigmine patches versus capsules for the treatment of Alzheimer disease. Neurology 2007, 69, 23–28. [Google Scholar] [CrossRef]
- Stott, P.W.; Williams, A.C.; Barry, B.W. Mechanistic study into the enhanced transdermal permeation of a model β-blocker, propranolol, by fatty acids a melting point depression effect. Int. J. Pharm. 2007, 219, 161–176. [Google Scholar] [CrossRef]
- Khan, M.Z.I.; Stedul, H.P.; Kerjakovic, N. A pH dependent colon targeted drug delivery system using methacrylate polymer. Drug Dev. Ind. Pharm. 2000, 26, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Pierre, M.B.R.; Ricci, E.; Tedesco, A.C.; Bentley, M.V.L.B. Oleic acid as optimizer of the skin delivery of 5-aminolevulinic acid in photodynamic therapy. Pharm. Res. 2006, 23, 360–366. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent advances in transdermal drug delivery systems: A review. Biomater. Res. 2021, 25, 1–15. [Google Scholar] [CrossRef]
- Sreenivas, S.A.; Pai, K.V. Thiolated Chitosans: Novel Polymers for Mucoadhesive Drug Delivery—A Review. Trop. J. Pharm. Res. 2008, 7, 1077–1088. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.; Fang, L.; Ling, L.; Wang, Q.; Liu, S.; Zhao, L.; He, Z. Design and in vivo evaluation of an indapamide transdermal patch. Int. J. Pharm. 2009, 370, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.; Dwivedi, S.; Giri, T.K.; Saraf, S.; Saraf, S.; Tripathi, D.K. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J. Control. Release 2012, 164, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, S.; Parekh, H.S.; Davies, N.M.; Nayanabhirama, U. A combined approach of chemical enhancers and sonophoresis for the transdermal delivery of tizanidine hydrochloride. Drug Deliv. 2009, 16, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Sudhakar, K.; Fuloria, S.; Subramaniyan, V.; Sathasivam, K.V.; Azad, A.K.; Swain, S.S.; Fuloria, N.K. Ultraflexible Liposome Nanocargo as a Dermal and Transdermal Drug Delivery System. Nanomaterials 2021, 11, 2557. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C.; Barry, B.W. Penetration enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.T.; Gerber, M.; Plessis, J.D.; Hamman, J.H. Transdermal drug delivery enhancement by compounds of natural origin. Molecules 2011, 16, 10507–10540. [Google Scholar] [CrossRef]
- Rajan, R.; Vasudevan, D.T. Effect of permeation enhancers on the penetration mechanism of transfersomal gel of ketoconazole. J. Adv. Pharm. Technol. Res. 2012, 3, 112. [Google Scholar]
- Kim, S.; Dangol, M.; Kang, G.; Lahiji, S.F.; Yang, H.; Jang, M.; Jung, H. Enhanced transdermal delivery by combined application of dissolving microneedle patch on serum-treated skin. Mol. Pharm. 2017, 14, 2024–2031. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhlaq, M.; Azad, A.K.; Fuloria, S.; Meenakshi, D.U.; Raza, S.; Safdar, M.; Nawaz, A.; Subramaniyan, V.; Sekar, M.; Sathasivam, K.V.; et al. Fabrication of Tizanidine Loaded Patches Using Flaxseed Oil and Coriander Oil as a Penetration Enhancer for Transdermal Delivery. Polymers 2021, 13, 4217. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234217
Akhlaq M, Azad AK, Fuloria S, Meenakshi DU, Raza S, Safdar M, Nawaz A, Subramaniyan V, Sekar M, Sathasivam KV, et al. Fabrication of Tizanidine Loaded Patches Using Flaxseed Oil and Coriander Oil as a Penetration Enhancer for Transdermal Delivery. Polymers. 2021; 13(23):4217. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234217
Chicago/Turabian StyleAkhlaq, Muhammad, Abul Kalam Azad, Shivkanya Fuloria, Dhanalekshmi Unnikrishnan Meenakshi, Sajid Raza, Muhammad Safdar, Asif Nawaz, Vetriselvan Subramaniyan, Mahendran Sekar, Kathiresan V. Sathasivam, and et al. 2021. "Fabrication of Tizanidine Loaded Patches Using Flaxseed Oil and Coriander Oil as a Penetration Enhancer for Transdermal Delivery" Polymers 13, no. 23: 4217. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234217