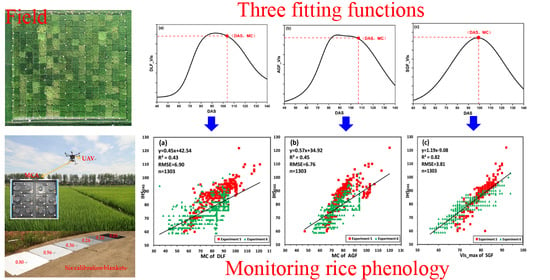
Monitoring Hybrid Rice Phenology at Initial Heading Stage Based on Low-Altitude Remote Sensing Data
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Sites
2.2. Experimental Design
2.3. Experimental Data
2.3.1. Meteorological Data
2.3.2. Field Phenological Data
2.3.3. Daily Reflectance
2.3.4. The Hyperspectral Reflectance
2.3.5. The UAV Multispectral Reflectance
2.4. Research Methods
2.4.1. Vegetation Indices (VIs)
2.4.2. Fitting Functions
2.4.3. The Process of Modeling
2.4.4. The Assessment of Models
3. Results
3.1. Statistical Analysis of Meteorological Data
3.2. Statistical Analysis of IHSDAS
3.3. Comparative Analysis of Daily NDVI and CIred Edge
3.4. Fitting Several Source CIred Edge
3.4.1. Fitting CIred Edge of SKYE
3.4.2. Fitting CIred Edge of ASD
3.4.3. Fitting CIred Edge of MCA
3.5. Monitoring IHSDAS Based on Several Source CIred Edge
3.5.1. Monitoring IHSDAS Based on CIred Edge of SKYE
3.5.2. Monitoring IHSDAS Based on CIred Edge of ASD
3.5.3. Monitoring IHSDAS Based on CIred Edge of MCA
3.6. Effects of Rice Cultivars and N Rates on IHSDAS
4. Discussion
4.1. Daily CIred Edge for Monitoring RP
4.2. Comparative Analysis of DLF, AGF and SGF for Fitting Several Source CIred Edge
4.3. Monitoring IHSDAS Based on Several Source CIred Edge
4.4. The Influence Factors on IHSDAS
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kuenzer, C.; Knauer, K. Remote sensing of rice crop areas. Int. J. Remote Sens. 2013, 34, 2101–2139. [Google Scholar] [CrossRef]
- Wang, H.; Deng, X.W. Development of the “Third-Generation” Hybrid Rice in China. Genom. Proteom. Bioinform. 2018, 16, 393–396. [Google Scholar] [CrossRef]
- Kempe, K.; Gils, M. Pollination control technologies for hybrid breeding. Mol. Breed. 2011, 27, 417–437. [Google Scholar] [CrossRef]
- Cheng, S.H.; Zhuang, J.Y.; Fan, Y.Y.; Du, J.H.; Cao, L.Y. Progress in research and development on hybrid rice: A super-domesticate in china. Ann. Bot. 2007, 100, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, S.; Gong, J.; Zhao, Y.; Feng, Q.; Gong, H.; Li, W.; Zhan, Q.; Cheng, B.; Xia, J.; et al. Genomic analysis of hybrid rice varieties reveals numerous superior alleles that contribute to heterosis. Nat. Commun. 2015, 6, 7258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, S. Dilemma and Way-out of Hybrid Rice during the Transition Period in China. Acta Agron. Sin. 2016, 42, 313–319. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, H.B.; Xu, X.Q.; He, J.Y.; Ge, X.K.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.X.; Tian, Y.C. Predicting grain yield in rice using multi-temporal vegetation indices from UAV-based multispectral and digital imagery. ISPRS J. Photogramm. Remote Sens. 2017, 130, 246–255. [Google Scholar] [CrossRef]
- Yang, Z.; Shao, Y.; Li, K.; Liu, Q.; Liu, L.; Brisco, B. An improved scheme for rice phenology estimation based on time-series multispectral HJ-1A/B and polarimetric RADARSAT-2 data. Remote Sens. Environ. 2017, 195, 184–201. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, T.; Yao, X.; Deng, X.; Tian, Y.; Cao, W.; Zhu, Y. Detection of rice phenology through time series analysis of ground-based spectral index data. Field Crops Res. 2016, 198, 131–139. [Google Scholar] [CrossRef]
- Li, J.; Zhou, J.W.; Xu, P.; Deng, X.N.; Deng, W.; Zhang, Y.; Yang, Y.; Tao, D.Y. Mapping five novel interspecific hybrid sterility loci between Oryza sativa and Oryza meridionalis. Breed. Sci. 2018, 68, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Guo, T.; Yu, X.; Li, X.; Zhang, H.; Zhu, C.; Flint-Garcia, S.; McMullen, M.D.; Holland, J.B.; Szalma, S.J.; Wisser, R.J.; et al. Optimal Designs for Genomic Selection in Hybrid Crops. Mol. Plant 2019, 12, 390–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Fukatsu, T.; Ninomiya, S. Automated characterization of flowering dynamics in rice using field-acquired time-series RGB images. Plant Methods 2015, 11, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Lan, Y.; Wang, J.; Chen, S.; Huang, C.; Liu, Q.; Liang, Q. Distribution law of rice pollen in the wind field of small UAV. Int. J. Agric. Biol. Eng. 2017, 10, 32–40. [Google Scholar] [CrossRef]
- Yabe, S.; Nakagawa, H.; Adachi, S.; Mukouyama, T.; Arai-Sanoh, Y.; Okamura, M.; Kondo, M.; Yoshida, H. Model analysis of genotypic difference in the variation of the duration from heading to flower opening based on the flower position on a panicle in high-yielding rice cultivars. Field Crops Res. 2018, 223, 155–163. [Google Scholar] [CrossRef]
- Wan, L.; Cen, H.; Zhu, J.; Zhang, J.; Zhu, Y.; Sun, D.; Du, X.; Zhai, L.; Weng, H.; Li, Y.; et al. Grain yield prediction of rice using multi-temporal UAV-based RGB and multispectral images and model transfer—A case study of small farmlands in the South of China. Agric. For. Meteorol. 2020, 291, 108096. [Google Scholar] [CrossRef]
- Piao, S.; Liu, Q.; Chen, A.; Janssens, I.A.; Fu, Y.; Dai, J.; Liu, L.; Lian, X.; Shen, M.; Zhu, X. Plant phenology and global climate change: Current progresses and challenges. Glob. Chang. Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wallach, D.; Liu, B.; Dingkuhn, M.; Boote, K.J.; Singh, U.; Asseng, S.; Kahveci, T.; He, J.; Zhang, R.; et al. Comparison of three calibration methods for modeling rice phenology. Agric. For. Meteorol. 2020, 280, 107785. [Google Scholar] [CrossRef]
- Weil, G.; Lensky, I.M.; Levin, N. Using ground observations of a digital camera in the VIS-NIR range for quantifying the phenology of Mediterranean woody species. Int. J. Appl. Earth Obs. Geoinf. 2017, 62, 88–101. [Google Scholar] [CrossRef]
- Gnyp, M.L.; Miao, Y.; Yuan, F.; Ustin, S.L.; Yu, K.; Yao, Y.; Huang, S.; Bareth, G. Hyperspectral canopy sensing of paddy rice aboveground biomass at different growth stages. Field Crops Res. 2014, 155, 42–55. [Google Scholar] [CrossRef]
- Zhang, S.; Tao, F. Modeling the response of rice phenology to climate change and variability in different climatic zones: Comparisons of five models. Eur. J. Agron. 2013, 45, 165–176. [Google Scholar] [CrossRef]
- Kim, M.; Ko, J.; Jeong, S.; Yeom, J.-m.; Kim, H.-o. Monitoring canopy growth and grain yield of paddy rice in South Korea by using the GRAMI model and high spatial resolution imagery. Gisci. Remote Sens. 2017, 54, 534–551. [Google Scholar] [CrossRef]
- Weir, A.H.; Bragg, P.L.; Porter, J.R.; Rayner, J.H. A winter-wheat crop simulation-model without water or nutrient limitations. J. Agric. Sci. 1984, 102, 371–382. [Google Scholar] [CrossRef]
- Wu, L.; Liu, X.; Zhou, B.; Li, L.; Tan, Z. Spatial-time continuous changes simulation of crop growth parameters with multi-source remote sensing data and crop growth model. J. Remote Sens. 2012, 16, 1173–1191. [Google Scholar]
- Villa, P.; Pinardi, M.; Bolpagni, R.; Gillier, J.-M.; Zinke, P.; Nedelcuţ, F.; Bresciani, M. Assessing macrophyte seasonal dynamics using dense time series of medium resolution satellite data. Remote Sens. Environ. 2018, 216, 230–244. [Google Scholar] [CrossRef]
- Zhang, X.; Friedl, M.A.; Schaaf, C.B.; Strahler, A.H.; Hodges, J.C.F.; Gao, F.; Reed, B.C.; Huete, A. Monitoring vegetation phenology using MODIS. Remote Sens. Environ. 2003, 84, 471–475. [Google Scholar] [CrossRef]
- Zhang, S.; Tao, F. Improving rice development and phenology prediction across contrasting climate zones of China. Agric. For. Meteorol. 2019, 268, 224–233. [Google Scholar] [CrossRef]
- Xie, Y.Y.; Civco, D.L.; Silander, J.A. Species-specific spring and autumn leaf phenology captured by time-lapse digital cameras. Ecosphere 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Meng, J.; Quackenbush, L.J. Use of remote sensing to predict the optimal harvest date of corn. Field Crops Res. 2019, 236, 1–13. [Google Scholar] [CrossRef]
- Ma, Y.; Fang, S.; Peng, Y.; Gong, Y.; Wang, D. Remote Estimation of Biomass in Winter Oilseed Rape (Brassica napus L.) Using Canopy Hyperspectral Data at Different Growth Stages. Appl. Sci. 2019, 9, 545. [Google Scholar] [CrossRef] [Green Version]
- Quan, X.; He, B.; Yebra, M.; Yin, C.; Liao, Z.; Zhang, X.; Li, X. A radiative transfer model-based method for the estimation of grassland aboveground biomass. Int. J. Appl. Earth Obs. Geoinf. 2017, 54, 159–168. [Google Scholar] [CrossRef]
- Vrieling, A.; Meroni, M.; Darvishzadeh, R.; Skidmore, A.K.; Wang, T.; Zurita-Milla, R.; Oosterbeek, K.; O’Connor, B.; Paganini, M. Vegetation phenology from Sentinel-2 and field cameras for a Dutch barrier island. Remote Sens. Environ. 2018, 215, 517–529. [Google Scholar] [CrossRef]
- Wang, Y.F.; Xue, Z.H.; Chen, J.; Chen, G.Z. Spatio-temporal analysis of phenology in Yangtze River Delta based on MODIS NDVI time series from 2001 to 2015. Front. Earth Sci. 2019, 13, 92–110. [Google Scholar] [CrossRef]
- Sakamoto, T.; Yokozawa, M.; Toritani, H.; Shibayama, M.; Ishitsuka, N.; Ohno, H. A crop phenology detection method using time-series MODIS data. Remote Sens. Environ. 2005, 96, 366–374. [Google Scholar] [CrossRef]
- Onojeghuo, A.O.; Blackburn, G.A.; Wang, Q.; Atkinson, P.M.; Kindred, D.; Miao, Y. Rice crop phenology mapping at high spatial and temporal resolution using downscaled MODIS time-series. GIScience Remote Sens. 2018, 55, 659–677. [Google Scholar] [CrossRef] [Green Version]
- Sun, N.; Wang, P.; Huang, F.; Li, B. Developing an integrated index based on phenological metrics for evaluating cadmium stress in rice using Sentinel-2 data. J. Appl. Remote Sens. 2018, 12, 036018. [Google Scholar] [CrossRef]
- Ricotta, C.; Avena, G.C. The remote sensing approach in broad-scale phenological studies. Appl. Veg. Sci. 2000, 3, 117–122. [Google Scholar] [CrossRef]
- White, M.A.; Hoffman, F.; Hargrove, W.W.; Nemani, R.R. A global framework for monitoring phenological responses to climate change. Geophys. Res. Lett. 2005, 32. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Magagi, R.; Goïta, K.; Trudel, M.; McNairn, H.; Powers, J. Crop phenology retrieval via polarimetric SAR decomposition and Random Forest algorithm. Remote Sens. Environ. 2019, 231, 111234. [Google Scholar] [CrossRef]
- Hirooka, Y.; Homma, K.; Maki, M.; Sekiguchi, K. Applicability of synthetic aperture radar (SAR) to evaluate leaf area index (LAI) and its growth rate of rice in farmers’ fields in Lao PDR. Field Crops Res. 2015, 176, 119–122. [Google Scholar] [CrossRef] [Green Version]
- Fikriyah, V.N.; Darvishzadeh, R.; Laborte, A.; Khan, N.I.; Nelson, A. Discriminating transplanted and direct seeded rice using Sentinel-1 intensity data. Int. J. Appl. Earth Obs. Geoinf. 2019, 76, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Sanchez, J.M.; Cloude, S.R.; Ballester-Berman, J.D. Rice Phenology Monitoring by Means of SAR Polarimetry at X-Band. IEEE Trans. Geosci. Remote Sens. 2012, 50, 2695–2709. [Google Scholar] [CrossRef]
- Yuzugullu, O.; Erten, E.; Hajnsek, I. Rice Growth Monitoring by Means of X-Band Co-polar SAR: Feature Clustering and BBCH Scale. IEEE Geosci. Remote Sens. Lett. 2015, 12, 1218–1222. [Google Scholar] [CrossRef]
- He, Z.; Li, S.H.; Wang, Y.; Dai, L.Y.; Lin, S. Monitoring Rice Phenology Based on Backscattering Characteristics of Multi-Temporal RADARSAT-2 Datasets. Remote Sens. 2018, 10, 340. [Google Scholar] [CrossRef] [Green Version]
- Bendig, J.; Yu, K.; Aasen, H.; Bolten, A.; Bennertz, S.; Broscheit, J.; Gnyp, M.L.; Bareth, G. Combining UAV-based plant height from crop surface models, visible, and near infrared vegetation indices for biomass monitoring in barley. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 79–87. [Google Scholar] [CrossRef]
- Graham, E.A.; Riordan, E.C.; Yuen, E.M.; Estrin, D.; Rundel, P.W. Public Internet-connected cameras used as a cross-continental ground-based plant phenology monitoring system. Glob. Chang. Biol. 2010, 16, 3014–3023. [Google Scholar] [CrossRef]
- Li, L.; Ren, T.; Ma, Y.; Wei, Q.; Wang, S.; Li, X.; Cong, R.; Liu, S.; Lu, J. Evaluating chlorophyll density in winter oilseed rape (Brassica napus L.) using canopy hyperspectral red-edge parameters. Comput. Electron. Agric. 2016, 126, 21–31. [Google Scholar] [CrossRef]
- Zhou, M.; Ma, X.; Wang, K.; Cheng, T.; Tian, Y.; Wang, J.; Zhu, Y.; Hu, Y.; Niu, Q.; Gui, L.; et al. Detection of phenology using an improved shape model on time-series vegetation index in wheat. Comput. Electron. Agric. 2020, 173, 105398. [Google Scholar] [CrossRef]
- Yang, Q.; Shi, L.; Han, J.; Yu, J.; Huang, K. A near real-time deep learning approach for detecting rice phenology based on UAV images. Agric. For. Meteorol. 2020, 287, 107938. [Google Scholar] [CrossRef]
- Bai, X.; Cao, Z.; Zhao, L.; Zhang, J.; Lv, C.; Li, C.; Xie, J. Rice heading stage automatic observation by multi-classifier cascade based rice spike detection method. Agric. For. Meteorol. 2018, 259, 260–270. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, F.-C.; Jing, Y.-S.; Jiang, X.-D.; Yang, S.-B.; Han, X.-M. Multi-Temporal Detection of Rice Phenological Stages Using Canopy Spectrum. Rice Sci. 2014, 21, 108–115. [Google Scholar] [CrossRef]
- Han, J.; Shi, L.; Yang, Q.; Huang, K.; Zha, Y.; Yu, J. Real-time detection of rice phenology through convolutional neural network using handheld camera images. Precis. Agric. 2020. [Google Scholar] [CrossRef]
- Zeng, L.; Wardlow, B.D.; Xiang, D.; Hu, S.; Li, D. A review of vegetation phenological metrics extraction using time-series, multispectral satellite data. Remote Sens. Environ. 2020, 237, 111511. [Google Scholar] [CrossRef]
- Baugh, W.M.; Groeneveld, D.P. Empirical proof of the empirical line. Int. J. Remote Sens. 2008, 29, 665–672. [Google Scholar] [CrossRef]
- Duan, B.; Liu, Y.; Gong, Y.; Peng, Y.; Wu, X.; Zhu, R.; Fang, S. Remote estimation of rice LAI based on Fourier spectrum texture from UAV image. Plant Methods 2019, 15. [Google Scholar] [CrossRef] [Green Version]
- Laliberte, A.S.; Goforth, M.A.; Steele, C.M.; Rango, A. Multispectral Remote Sensing from Unmanned Aircraft: Image Processing Workflows and Applications for Rangeland Environments. Remote Sens. 2011, 3, 2529–2551. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Mao, Z.; Li, X.; Hu, Z.; Duan, F.; Yan, Y. UAV-based multispectral remote sensing for precision agriculture: A comparison between different cameras. ISPRS J. Photogramm. Remote Sens. 2018, 146, 124–136. [Google Scholar] [CrossRef]
- Haiying, L.; Hongchun, Z. Hyperspectral characteristic analysis for leaf nitrogen content in different growth stages of winter wheat. Appl. Opt. 2016, 55, D151–D161. [Google Scholar] [CrossRef]
- Rouse, J.W., Jr.; Haas, R.; Schell, J.; Deering, D. Monitoring Vegetation Systems in the Great Plains with ERTS; NASA/GSFCT Type III Final Report; National Aeronautics and Space Administration (NASA): Washington, DC, USA, 1974; pp. 309–317.
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Minoli, S.; Egli, D.B.; Rolinski, S.; Müller, C. Modelling cropping periods of grain crops at the global scale. Glob. Planet. Chang. 2019, 174, 35–46. [Google Scholar] [CrossRef]
- Ishikawa, D.; Hoogenboom, G.; Hakoyama, S.; Ishiguro, E. A potential of the growth stage estimation for paddy rice by using chlorophyll absorption bands in the 400–1100 nm region. J. Agric. Meteorol. 2015, 71, 24–31. [Google Scholar] [CrossRef] [Green Version]
- De Castro, I.A.; Six, J.; Plant, E.R.; Peña, M.J. Mapping Crop Calendar Events and Phenology-Related Metrics at the Parcel Level by Object-Based Image Analysis (OBIA) of MODIS-NDVI Time-Series: A Case Study in Central California. Remote Sens. 2018, 10, 1745. [Google Scholar] [CrossRef] [Green Version]
- Kandasamy, S.; Baret, F.; Verger, A.; Neveux, P.; Weiss, M. A comparison of methods for smoothing and gap filling time series of remote sensing observations-application to MODIS LAI products. Biogeosciences 2013, 10, 4055–4071. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, P.; Eklundh, L. Seasonality extraction by function fitting to time-series of satellite sensor data. IEEE Trans. Geosci. Remote Sens. 2002, 40, 1824–1832. [Google Scholar] [CrossRef]
- Atkinson, P.M.; Jeganathan, C.; Dash, J.; Atzberger, C. Inter-comparison of four models for smoothing satellite sensor time-series data to estimate vegetation phenology. Remote Sens. Environ. 2012, 123, 400–417. [Google Scholar] [CrossRef]
- Beck, P.S.A.; Atzberger, C.; Hogda, K.A.; Johansen, B.; Skidmore, A.K. Improved monitoring of vegetation dynamics at very high latitudes: A new method using MODIS NDVI. Remote Sens. Environ. 2006, 100, 321–334. [Google Scholar] [CrossRef]
- Hird, J.N.; McDermid, G.J. Noise reduction of NDVI time series: An empirical comparison of selected techniques. Remote Sens. Environ. 2009, 113, 248–258. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Henebry, G.M. Impacts of land cover and land use change on long-term trend of land surface phenology: A case study in agricultural ecosystems. Environ. Res. Lett. 2019, 14, 044020. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, P.; Eklundh, L. TIMESAT—A program for analyzing time-series of satellite sensor data. Comput. Geosci. 2004, 30, 833–845. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Won, P.L.P.; Banayo, N.P.M.; Nie, L.; Peng, S.; Kato, Y. Late-season nitrogen applications improve grain yield and fertilizer-use efficiency of dry direct-seeded rice in the tropics. Field Crops Res. 2019, 233, 114–120. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Y.; Meng, L.; Asrar, G.; Sapkota, A.; Coates, F. Characterizing the relationship between satellite phenology and pollen season: A case study of birch. Remote Sens. Environ. 2019, 222, 267–274. [Google Scholar] [CrossRef]
- Haerani, H.; Apan, A.; Basnet, B. Mapping of peanut crops in Queensland, Australia, using time-series PROBA-V 100-m normalized difference vegetation index imagery. J. Appl. Remote Sens. 2018, 12, 036005. [Google Scholar] [CrossRef]
- Wang, C.; Feng, M.-C.; Yang, W.-D.; Ding, G.-W.; Sun, H.; Liang, Z.-Y.; Xie, Y.-K.; Qiao, X.-X. Impact of spectral saturation on leaf area index and aboveground biomass estimation of winter wheat. Spectrosc. Lett. 2016, 49, 241–248. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Peng, Y.; Vina, A.; Arkebauer, T.; Schepers, J.S. Efficiency of chlorophyll in gross primary productivity: A proof of concept and application in crops. J. Plant Physiol. 2016, 201, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanning, M.; Kühling, I.; Trautz, D.; Jarmer, T. High-Resolution UAV-Based Hyperspectral Imagery for LAI and Chlorophyll Estimations from Wheat for Yield Prediction. Remote Sens. 2018, 10, 2000. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Lenz-Wiedemann, V.; Chen, X.; Bareth, G. Estimating leaf chlorophyll of barley at different growth stages using spectral indices to reduce soil background and canopy structure effects. ISPRS J. Photogramm. Remote Sens. 2014, 97, 58–77. [Google Scholar] [CrossRef]
- Sun, T.; Fang, H.; Liu, W.; Ye, Y. Impact of water background on canopy reflectance anisotropy of a paddy rice field from multi-angle measurements. Agric. For. Meteorol. 2017, 233, 143–152. [Google Scholar] [CrossRef]
- Din, M.; Zheng, W.; Rashid, M.; Wang, S.; Shi, Z. Evaluating Hyperspectral Vegetation Indices for Leaf Area Index Estimation of Oryza sativa L. at Diverse Phenological Stages. Front. Plant Sci. 2017, 8, 820. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.; Yang, Y.; Chen, X.; Yu, X.; Wu, Y.; Xiong, F. Effects of high temperature during two growth stages on caryopsis development and physicochemical properties of starch in rice. Int. J. Biol. Macromol. 2020, 145, 301–310. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, W.; Ding, Y.; Liu, G.; Xing, W.; Cao, X.; Chen, D. Assessment of climate change impact on the water footprint in rice production: Historical simulation and future projections at two representative rice cropping sites of China. Sci. Total Environ. 2020, 709, 136190. [Google Scholar] [CrossRef]
- Wang, H.; Ghosh, A.; Linquist, B.A.; Hijmans, R.J. Satellite-Based Observations Reveal Effects of Weather Variation on Rice Phenology. Remote Sens. 2020, 12, 1522. [Google Scholar] [CrossRef]
- Liu, J.T.; Feng, Q.L.; Gong, J.H.; Zhou, J.P.; Liang, J.M.; Li, Y. Winter wheat mapping using a random forest classifier combined with multi-temporal and multi-sensor data. Int. J. Digit. Earth 2018, 11, 783–802. [Google Scholar] [CrossRef]
- Hufkens, K.; Melaas, E.K.; Mann, M.L.; Foster, T.; Ceballos, F.; Robles, M.; Kramer, B. Monitoring crop phenology using a smartphone based near-surface remote sensing approach. Agric. For. Meteorol. 2019, 265, 327–337. [Google Scholar] [CrossRef]
- Hassan, M.A.; Yang, M.; Rasheed, A.; Yang, G.; Reynolds, M.; Xia, X.; Xiao, Y.; He, Z. A rapid monitoring of NDVI across the wheat growth cycle for grain yield prediction using a multi-spectral UAV platform. Plant Sci. 2018. [Google Scholar] [CrossRef]
- Nguy-Robertson, A.; Gitelson, A.; Peng, Y.; Walter-Shea, E.; Leavitt, B.; Arkebauer, T. Continuous monitoring of crop reflectance, vegetation fraction, and identification of developmental stages using a four band radiometer. Agron. J. 2013, 105, 1769–1779. [Google Scholar] [CrossRef] [Green Version]
- Candiago, S.; Remondino, F.; De Giglio, M.; Dubbini, M.; Gattelli, M. Evaluating Multispectral Images and Vegetation Indices for Precision Farming Applications from UAV Images. Remote Sens. 2015, 7, 4026–4047. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Chen, S.-Y.; Chen, C.-C.; Tai, C.-H. Detecting newly grown tree leaves from unmanned-aerial-vehicle images using hyperspectral target detection techniques. ISPRS J. Photogramm. Remote Sens. 2018, 142, 174–189. [Google Scholar] [CrossRef]
- Mathews, A.J.; Jensen, J.L.R. Visualizing and Quantifying Vineyard Canopy LAI Using an Unmanned Aerial Vehicle (UAV) Collected High Density Structure from Motion Point Cloud. Remote Sens. 2013, 5, 2164–2183. [Google Scholar] [CrossRef] [Green Version]
- Czernecki, B.; Nowosad, J.; Jablonska, K. Machine learning modeling of plant phenology based on coupling satellite and gridded meteorological dataset. Int. J. Biometeorol. 2018, 62, 1297–1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araya, S.; Ostendorf, B.; Lyle, G.; Lewis, M. CropPhenology: An R package for extracting crop phenology from time series remotely sensed vegetation index imagery. Ecol. Inform. 2018, 46, 45–56. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Cao, Q.; Zhu, Y.; Tang, L.; Rehmani, M.I.A.; Cao, W. Non-destructive Assessment of Plant Nitrogen Parameters Using Leaf Chlorophyll Measurements in Rice. Front. Plant Sci. 2016, 7, 1829. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chang, Q.; Li, F.; Yan, L.; Huang, Y.; Wang, Q.; Luo, L. Effects of Growth Stage Development on Paddy Rice Leaf Area Index Prediction Models. Remote Sens. 2019, 11, 361. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Huang, Y.; Sun, W.; Yu, L. Shifts in cultivar and planting date have regulated rice growth duration under climate warming in China since the early 1980s. Agric. For. Meteorol. 2017, 247, 34–41. [Google Scholar] [CrossRef]
- Peng, S.B.; Huang, J.L.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.H.; Centeno, G.S.; Khush, G.S.; Cassman, K.G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julia, C.; Dingkuhn, M. Predicting temperature induced sterility of rice spikelets requires simulation of crop-generated microclimate. Eur. J. Agron. 2013, 49, 50–60. [Google Scholar] [CrossRef]


















| Experiment | Year and Study Site | Number of Plots | Plots Area (m2) | Sowing Time (Month/Day/Year) | Number of Rice Cultivars | N Rates (kg/hm2) |
|---|---|---|---|---|---|---|
| 1 | 2016 Ezhou | 16 | 40 | 5/7/2016 | 16 | 180 |
| 2 | 2017 Ezhou | 52 | 40 | 5/7/2017 | 52 | 180 |
| 3 | 2017–2018 Lingshui | 40 | 40 | 12/10/2017 | 40 | 180 |
| 4 | 2017–2018 Lingshui | 24 | 15 | 12/10/2017 | 2 | 0, 120, 180 and 240 |
| 5 | 2018 Ezhou | 1014 | 1 | 5/25/2018 | 1014 | 180 |
| 6 | 2019 Ezhou | 289 | 1 | 5/15/2019 | 289 | 180 |
| SKYE Radiometers | URS | DRS | ||
|---|---|---|---|---|
| Central Wavelength (nm) | Band Width (nm) | Central Wavelength (nm) | Band Width (nm) | |
| A_SKYE | 550.3 | 39.9 | 549.7 | 39.8 |
| 655.1 | 34.6 | 655.3 | 34.7 | |
| 717.2 | 23.7 | 716.8 | 23.6 | |
| 865.6 | 28.5 | 865.0 | 31.5 | |
| B_SKYE | 550.0 | 39.9 | 549.9 | 38.7 |
| 655.1 | 36.7 | 654.9 | 36.0 | |
| 716.6 | 23.6 | 717.7 | 23.9 | |
| 865.3 | 27.9 | 865.7 | 29.7 | |
| C_SKYE | 551.8 | 39.6 | 549.5 | 39.7 |
| 652.9 | 35.7 | 653.4 | 35.9 | |
| 717.0 | 26.2 | 715.8 | 25.2 | |
| 857.4 | 38.0 | 858.3 | 37.8 | |
| Experiment | Year and Study Site | SKYE Radiometers | Rice Cultivars | IHSDAS | N Rates (kg/hm2) |
|---|---|---|---|---|---|
| 1 | 2016 Ezhou | C_SKYE | LY 9348 | 97 | 180 |
| 2 | 2017 Ezhou | A_SKYE | FLY 4 | 85 | 180 |
| B_SKYE | YLYHZ | 77 | 180 | ||
| C_SKYE | LY 9348 | 87 | 180 | ||
| 4 | 2017–2018 Lingshui | A_SKYE | LY 9348 | 106 | 120 |
| B_SKYE | FLY 4 | 109 | 120 | ||
| C_SKYE | LY 9348 | 109 | 240 | ||
| 6 | 2019 Ezhou | A_SKYE | FLY 4 | 89 | 180 |
| B_SKYE | LY 9348 | 91 | 180 |
| Experiment | Year and Study Site | Measurement Dates (Month/Day/Year) | Days after Sowing (DAS) | Number of Rice Cultivars |
|---|---|---|---|---|
| 1 | 2016 Ezhou | 7/28/2016 | 82 | 16 |
| 8/11/2016 | 96 | 16 | ||
| 9/2/2016 | 118 | 16 | ||
| 9/21/2016 | 137 | 16 | ||
| 2 | 2017 Ezhou | 6/20/2017 | 44 | 52 |
| 7/7/2017 | 61 | 52 | ||
| 7/18/2017 | 72 | 52 | ||
| 8/6/2017 | 91 | 52 | ||
| 8/23/2017 | 108 | 52 | ||
| 9/12/2017 | 128 | 52 | ||
| 3 | 2017–2018 Lingshui | 2/4/2018 | 56 | 40 |
| 2/25/2018 | 77 | 40 | ||
| 3/9/2018 | 89 | 40 | ||
| 3/19/2018 | 99 | 40 | ||
| 3/31/2018 | 111 | 40 | ||
| 4/28/2018 | 138 | 40 | ||
| 4 | 2017–2018 Lingshui | 2/4/2018 | 56 | 24 |
| 2/22/2018 | 74 | 24 | ||
| 3/4/2018 | 84 | 24 | ||
| 3/14/2018 | 94 | 24 | ||
| 3/24/2018 | 104 | 24 | ||
| 4/4/2018 | 115 | 24 | ||
| 4/29/2018 | 140 | 24 |
| Band Number | Center Wavelength (nm) | Bandwidth (nm) | Band Number | Center Wavelength (nm) | Bandwidth (nm) |
|---|---|---|---|---|---|
| 1 | 490 | 10 | 7 | 700 | 10 |
| 2 | 520 | 10 | 8 | 720 | 10 |
| 3 | 550 | 10 | 9 | 800 | 10 |
| 4 | 570 | 10 | 10 | 850 | 10 |
| 5 | 670 | 10 | 11 | 900 | 20 |
| 6 | 680 | 10 | 12 | 950 | 40 |
| Experiment | Year and Study Site | Measurement Dates (Month/Day/Year) | Days after Sowing (DAS) | Number of Rice Cultivars |
|---|---|---|---|---|
| 5 | 2018 Ezhou | 6/27/2018 | 33 | 1014 |
| 7/6/2018 | 42 | 1014 | ||
| 7/11/2018 | 47 | 1014 | ||
| 7/16/2018 | 52 | 1014 | ||
| 7/27/2018 | 63 | 1014 | ||
| 8/2/2018 | 69 | 1014 | ||
| 8/9/2018 | 76 | 1014 | ||
| 8/15/2018 | 82 | 1014 | ||
| 8/21/2018 | 88 | 1014 | ||
| 8/27/2018 | 94 | 1014 | ||
| 6 | 2019 Ezhou | 2/7/2019 | 42 | 289 |
| 6/7/2019 | 48 | 289 | ||
| 14/7/2019 | 52 | 289 | ||
| 22/7/2019 | 60 | 289 | ||
| 27/7/2019 | 68 | 289 | ||
| 1/8/2019 | 73 | 289 | ||
| 6/8/2019 | 78 | 289 | ||
| 11/8/2019 | 83 | 289 | ||
| 16/8/2019 | 88 | 289 | ||
| 22/8/2019 | 93 | 289 | ||
| 29/8/2019 | 99 | 289 | ||
| 3/9/2019 | 106 | 289 | ||
| 9/9/2019 | 111 | 289 | ||
| 17/9/2019 | 117 | 289 |
| Experiment | Number of Plots | Minimum | Mean | Maximum | Standard Deviation | Variance | Coefficient of Variation (%) |
|---|---|---|---|---|---|---|---|
| 1 | 16 | 92 | 98.31 | 104 | 3.45 | 11.96 | 3.51 |
| 2 | 52 | 76 | 84.00 | 94 | 5.59 | 31.29 | 6.66 |
| 3 | 40 | 96 | 110.60 | 119 | 3.71 | 13.73 | 3.35 |
| 4 | 24 | 103 | 106.58 | 110 | 2.15 | 4.60 | 2.01 |
| 5 | 1014 | 59 | 76.38 | 103 | 7.44 | 55.40 | 9.74 |
| 6 | 289 | 60 | 87.42 | 122 | 9.27 | 85.84 | 10.60 |
| Models | Fitting Function | R2 | RMSE | Fitting Function | R2 | RMSE | Fitting Function | R2 | RMSE |
|---|---|---|---|---|---|---|---|---|---|
| a | DLF | 0.97 | 0.07 | AGF | 0.97 | 0.07 | SGF | 0.92 | 0.13 |
| b | 0.94 | 0.19 | 0.96 | 0.14 | 0.88 | 0.27 | |||
| c | 0.96 | 0.13 | 0.96 | 0.14 | 0.94 | 0.16 | |||
| d | 0.94 | 0.17 | 0.95 | 0.16 | 0.91 | 0.22 | |||
| e | 0.98 | 0.13 | 0.98 | 0.14 | 0.95 | 0.23 | |||
| f | 0.98 | 0.14 | 0.98 | 0.13 | 0.92 | 0.36 | |||
| g | 0.98 | 0.14 | 0.98 | 0.15 | 0.95 | 0.28 | |||
| h | 0.97 | 0.14 | 0.96 | 0.15 | 0.89 | 0.26 | |||
| i | 0.96 | 0.15 | 0.97 | 0.14 | 0.89 | 0.25 |
| Experiment | Number of Plots | Fitting Functions | R2 | RMSE | ||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Mean | Maximum | Minimum | Mean | Maximum | |||
| 1 | n = 16 | DLF | 0.95 | 0.99 | 1 | 0 | 0.02 | 0.09 |
| AGF | 1 | 1 | 1 | 0 | 0 | 0 | ||
| SGF | 0.91 | 0.97 | 0.99 | 0.004 | 0.06 | 0.11 | ||
| 2 | n = 52 | DLF | 0.84 | 0.98 | 1 | 0 | 0.05 | 0.18 |
| AGF | 0.25 | 0.90 | 0.99 | 0.005 | 0.12 | 0.44 | ||
| SGF | 0.62 | 0.89 | 0.98 | 0.08 | 0.16 | 0.29 | ||
| 3 | n = 40 | DLF | 0.98 | 0.99 | 1 | 0 | 0.02 | 0.07 |
| AGF | 0.92 | 0.98 | 0.99 | 0.01 | 0.08 | 0.19 | ||
| SGF | 0.92 | 0.97 | 0.99 | 0.03 | 0.09 | 0.20 | ||
| 4 | n = 24 | DLF | 0.97 | 0.99 | 1 | 0 | 0.03 | 0.12 |
| AGF | 0.96 | 0.98 | 0.99 | 0.008 | 0.10 | 0.16 | ||
| SGF | 0.95 | 0.97 | 0.99 | 0.07 | 0.12 | 0.17 | ||
| Experiment | Number of Plots | Fitting Functions | R2 | RMSE | ||||
|---|---|---|---|---|---|---|---|---|
| Minimum | Mean | Maximum | Minimum | Mean | Maximum | |||
| 5 | n = 1014 | DLF | 0.18 | 0.95 | 0.99 | 0.015 | 0.13 | 0.86 |
| AGF | 0.32 | 0.92 | 0.99 | 0.02 | 0.19 | 0.90 | ||
| SGF | 0.32 | 0.90 | 0.99 | 0.04 | 0.23 | 0.96 | ||
| 6 | n = 289 | DLF | 0.69 | 0.96 | 0.99 | 0.05 | 0.16 | 0.34 |
| AGF | 0.01 | 0.94 | 0.99 | 0.08 | 0.20 | 1.27 | ||
| SGF | 0.24 | 0.91 | 0.98 | 0.15 | 0.27 | 0.47 | ||
| Experiment | Variable | Factor | Degree of Freedom | Sum of Squares | Mean Square | F Value |
|---|---|---|---|---|---|---|
| 4 | IHSDAS | Rice Cultivars | 1 | 1.50 | 1.50 | 5.14 * |
| IHSDAS | N rates | 3 | 95.17 | 31.72 | 108.76 ** | |
| IHSDAS | Rice Cultivars × N rates | 3 | 4.50 | 1.50 | 5.14 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Jiang, Q.; Wu, X.; Zhu, R.; Gong, Y.; Peng, Y.; Duan, B.; Fang, S. Monitoring Hybrid Rice Phenology at Initial Heading Stage Based on Low-Altitude Remote Sensing Data. Remote Sens. 2021, 13, 86. https://0-doi-org.brum.beds.ac.uk/10.3390/rs13010086
Ma Y, Jiang Q, Wu X, Zhu R, Gong Y, Peng Y, Duan B, Fang S. Monitoring Hybrid Rice Phenology at Initial Heading Stage Based on Low-Altitude Remote Sensing Data. Remote Sensing. 2021; 13(1):86. https://0-doi-org.brum.beds.ac.uk/10.3390/rs13010086
Chicago/Turabian StyleMa, Yi, Qi Jiang, Xianting Wu, Renshan Zhu, Yan Gong, Yi Peng, Bo Duan, and Shenghui Fang. 2021. "Monitoring Hybrid Rice Phenology at Initial Heading Stage Based on Low-Altitude Remote Sensing Data" Remote Sensing 13, no. 1: 86. https://0-doi-org.brum.beds.ac.uk/10.3390/rs13010086