Contact-Based Methods for Measuring Respiratory Rate
Abstract
:1. Introduction
1.1. The Importance of Respiratory Rate Monitoring
1.1.1. Clinical Settings
1.1.2. Occupational Settings
1.1.3. Sport and Exercise
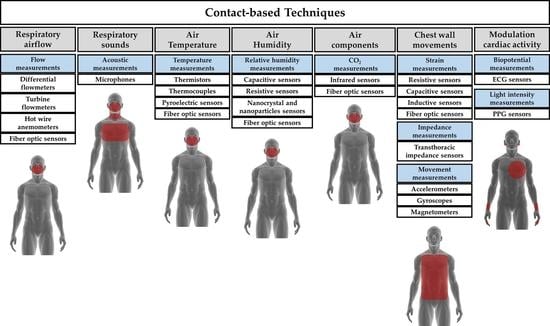
1.2. Taxonomy of Available Techniques for Respiratory Rate Monitoring
- Size (i.e., the size of the sensor used to collect the physical/chemical quantity);
- Cost (including an estimate of the cost of signal conditioning electronics);
- Real-time monitoring: ability to record the respiratory signal (and values) in real time;
- Measurement intrusiveness: how the sensor or the measuring technique limits the subject’s activity and movements;
- Sensitivity to body motion artifacts: sensitivity of a measuring technique to movements and motions not related to breathing that negatively affect the output signal;
- Influence of environmental factors: influence of temperature, humidity, external strains and other environmental factors that can affect sensor measurement and consequently the sensor output;
- Presence of wire: presence of tube, wires, and connections needed to supply the sensors, and/or register the physical/chemical quantity, and/or transfer the data for processing.
2. Techniques Based on Respiratory Airflow
2.1. Flow Sensors
2.1.1. Differential Flowmeters
- Pneumotachographs. They can be subdivided into Fleisch, where the resistance consists of capillary tubes [38], and into Lilly, where the resistance is a fine wire mesh [39]. In both cases, Hagen-Poiseuille law may express the linear relationship between the output () and the input (Q):being the dynamic viscosity of the gas, and L, r, and n the length, the radius, and the number of the capillary, respectively. Although quadratic models have been investigated [40,41], the linear relationship (Equation (1)) is mostly used for pneumotachographs. Linearity and a good frequency response (response time in the order of tens of ms) are two key factors for the use of this flowmeter in respiratory monitoring, if an adequate differential pressure sensor is chosen. One potential concern is related to the influence of the gas composition and the temperature on the pneumotachograph response because these factors affect the value of in Equation (1). Nevertheless, this aspect is relevant for accurate flow measurements, while it can be neglected for the estimation of .
- Orifice meters. They can be split into fixed orifice meters, where the resistance is an orifice plate, and into variable orifice meters, where the plate composing the resistance increases its passage area with flowrate (e.g., it consists of a flexible flap [42,43]). In both cases, the input-output relationship ( vs. Q) may be expressed as follows:where is the flowrate calculated considering ideal conditions, d is the diameter of the orifice, the ratio between the diameter of the orifice and the internal diameter of the pipe, and the gas density. Therefore, the input-output relationship is not linear for fixed orifice meter; conversely, the increase of the passage area with flow in the variable orifice meter has the effect of linearizing the input-output relationship. As for pneumotachographs, they have a good frequency response when an adequate differential pressure sensor is chosen.
2.1.2. Turbine Flowmeters
2.1.3. Hot Wire Anemometers
2.1.4. Fiber-Optic Based Flowmeters
2.2. Short Summary
3. Techniques Based on Respiratory Sounds
3.1. Acoustic Sensors
Microphones
3.2. Short Summary
4. Techniques Based on Air Temperature
4.1. Temperature Sensors
4.1.1. Thermistors
4.1.2. Thermocouples
4.1.3. Pyroelectric Sensors
4.1.4. Fiber-Optic Sensors
4.2. Short Summary
5. Techniques Based on Air Humidity
5.1. Humidity Sensors
5.1.1. Capacitive Sensors
5.1.2. Resistive Sensors
5.1.3. Nanocrystals and Nanoparticles Sensors
5.1.4. Fiber-Optic Sensors
5.2. Short Summary
6. Techniques Based on Air Components
6.1. CO2 Sensors
6.1.1. Infrared Sensors
6.1.2. Fiber-Optic Sensors
6.2. Short Summary
7. Techniques Based on Chest Wall Movement Analysis
7.1. Strain Sensors
7.1.1. Resistive Sensors
7.1.2. Capacitive Sensors
7.1.3. Inductive Sensors
7.1.4. Fiber-Optic Sensors
7.2. Impedance Sensors
Transthoracic Impedance Sensors
7.3. Movement Sensors
7.3.1. Accelerations Sensors (Accelerometers)
7.3.2. Angular Velocities Sensors (Gyroscopes)
7.3.3. Magnetic Field Sensors (Magnetometers)
7.4. Short Summary
8. Techniques Based on the Modulation of Cardiac Activity
8.1. Biopotential Sensors
ECG Sensors
8.2. Light Intensity Sensors
PPG Sensors
8.3. Short Summary
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Respiratory frequency | |
| bpm | Breaths per minute |
| SB | Slow breathing |
| QB | Quiet breathing |
| FB | Fast breathing |
| FOS | Fiber-optic sensor |
| DF | Differential flowmeter |
| HWA | Hot wire anemometer |
| FBG | Fiber Bragg Grating |
| Q | Airflow |
| P | Pressure |
| ΔP | Pressure drop |
| i | Current |
| R | Resistance |
| Wavelength | |
| I | Light intensity |
| V | Voltage |
| FEV1 | Forced expiratory volume in the 1st second |
| FVC | Forced vital capacity |
| T | Temperature |
| CORSA | Computerized Respiratory Sound Analysis |
| C | Capacitance |
| E | Applied voltage |
| CO2 | Carbon dioxide |
| MOD | Mean of differences |
| LOA | Limit of agreement |
| Bragg wavelength | |
| RH | Relative humidity |
| ppm | Parts per million |
| NDIR | Nondispersive infrared |
| COPD | Chronic obstructive pulmonary disease |
| Z | Impedance |
| MEMS | Mechanical and micro-electromechanical system |
| IMU | Inertial Measurement Unit |
| PPG | Photoplethysmography |
| ECG | Electrocardiography |
| EDR | ECG-derived respiration |
| RSA | Respiratory sinus arrhythmia |
| LED | Light-emitting diodes |
| PD | Photodetector |
References
- Nicolò, A.; Girardi, M.; Sacchetti, M. Control of the depth and rate of breathing: Metabolic vs. non-metabolic inputs. J. Physiol. 2017, 595, 6363–6364. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, A.; Girardi, M.; Bazzucchi, I.; Felici, F.; Sacchetti, M. Respiratory frequency and tidal volume during exercise: Differential control and unbalanced interdependence. Physiol. Rep. 2018, 6, e13908. [Google Scholar] [CrossRef] [PubMed]
- Tipton, M.J.; Harper, A.; Paton, J.F.; Costello, J.T. The human ventilatory response to stress: Rate or depth? J. Physiol. 2017, 595, 5729–5752. [Google Scholar] [CrossRef] [PubMed]
- Cretikos, M.A.; Bellomo, R.; Hillman, K.; Chen, J.; Finfer, S.; Flabouris, A. Respiratory rate: The neglected vital sign. Med. J. Aust. 2008. [Google Scholar] [CrossRef]
- Lovett, P.B.; Buchwald, J.M.; Stürmann, K.; Bijur, P. The vexatious vital: Neither clinical measurements by nurses nor an electronic monitor provides accurate measurements of respiratory rate in triage. Ann. Emerg. Med. 2005, 45, 68–76. [Google Scholar] [CrossRef]
- Folke, M.; Cernerud, L.; Ekström, M.; Hök, B. Critical review of non-invasive respiratory monitoring in medical care. Med. Biol. Eng. Comput. 2003, 41, 377–383. [Google Scholar] [CrossRef]
- AL-Khalidi, F.Q.; Saatchi, R.; Burke, D.; Elphick, H.; Tan, S. Respiration rate monitoring methods: A review. Pediatr. Pulmonol. 2011, 46, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Nicolò, A.; Massaroni, C.; Passfield, L. Respiratory frequency during exercise: The neglected physiological measure. Front. Physiol. 2017, 8, 922. [Google Scholar] [CrossRef]
- Parkes, R. Rate of respiration: The forgotten vital sign. Emerg. Nurse 2011, 19, 12–19. [Google Scholar] [CrossRef]
- Smith, I.; Mackay, J.; Fahrid, N.; Krucheck, D. Respiratory rate measurement: A comparison of methods. Br. J. Healthc. Assist. 2011, 5, 18–23. [Google Scholar] [CrossRef]
- Barthel, P.; Wensel, R.; Bauer, A.; Müller, A.; Wolf, P.; Ulm, K.; Huster, K.M.; Francis, D.P.; Malik, M.; Schmidt, G. Respiratory rate predicts outcome after acute myocardial infarction: A prospective cohort study. Eur. Heart J. 2013. [Google Scholar] [CrossRef]
- Helfenbein, E.; Firoozabadi, R.; Chien, S.; Carlson, E.; Babaeizadeh, S. Development of three methods for extracting respiration from the surface ECG: A review. J. Electrocardiol. 2014, 47, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Prasad, A.; Nagappa, M.; Wong, J.; Abrahamyan, L.; Chung, F.F. Risk factors for opioid-induced respiratory depression and failure to rescue: A review. Curr. Opin. Anaesthesiol. 2018, 31, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Rantonen, T.; Jalonen, J.; Grönlund, J.; Antila, K.; Southall, D.; Välimäki, I. Increased amplitude modulation of continuous respiration precedes sudden infant death syndrome: Detection by spectral estimation of respirogram. Early Hum. Dev. 1998, 53, 53–63. [Google Scholar] [CrossRef]
- Philip, K.; Richardson, R.; Cohen, M. Staff perceptions of respiratory rate measurement in a general hospital. Br. J. Nurs. 2013, 22, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Subbe, C.; Kinsella, S. Continuous Monitoring of Respiratory Rate in Emergency Admissions: Evaluation of the RespiraSense™ Sensor in Acute Care Compared to the Industry Standard and Gold Standard. Sensors 2018, 18, 2700. [Google Scholar] [CrossRef] [PubMed]
- Coca, A.; Roberge, R.J.; Jon Williams, W.; Landsittel, D.P.; Powell, J.B.; Palmiero, A. Physiological monitoring in firefighter ensembles: Wearable plethysmographic sensor vest versus standard equipment. J. Occup. Environ. Hyg. 2009. [Google Scholar] [CrossRef]
- Marcel-Millet, P.; Ravier, G.; Grospretre, S.; Gimenez, P.; Freidig, S.; Groslambert, A. Physiological responses and parasympathetic reactivation in rescue interventions: The effect of the breathing apparatus. Scand. J. Med. Sci. Sports 2018, 28, 2710–2722. [Google Scholar] [CrossRef]
- Grassmann, M.; Vlemincx, E.; von Leupoldt, A.; Mittelstädt, J.M.; Van den Bergh, O. Respiratory changes in response to cognitive load: A systematic review. Neural Plast. 2016, 2016, 8146809. [Google Scholar] [CrossRef]
- Grassmann, M.; Vlemincx, E.; von Leupoldt, A.; Van den Bergh, O. The role of respiratory measures to assess mental load in pilot selection. Ergonomics 2016. [Google Scholar] [CrossRef]
- White, M.D. Components and mechanisms of thermal hyperpnea. J. Appl. Physiol. 2006, 101, 655–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carballo-Leyenda, B.; Villa, J.G.; López-Satué, J.; Collado, P.S.; Rodríguez-Marroyo, J.A. Fractional contribution of wildland firefighters’ personal protective equipment on physiological strain. Front. Physiol. 2018. [Google Scholar] [CrossRef]
- Nicolò, A.; Bazzucchi, I.; Haxhi, J.; Felici, F.; Sacchetti, M. Comparing continuous and intermittent exercise: An “isoeffort” and “isotime” approach. PLoS ONE 2014, 9, e94990. [Google Scholar] [CrossRef]
- Nicolò, A.; Marcora, S.M.; Sacchetti, M. Respiratory frequency is strongly associated with perceived exertion during time trials of different duration. J. Sports Sci. 2016, 34, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, A.; Marcora, S.M.; Bazzucchi, I.; Sacchetti, M. Differential control of respiratory frequency and tidal volume during high-intensity interval training. Exp. Physiol. 2017, 102, 934–949. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, A.; Bazzucchi, I.; Lenti, M.; Haxhi, J.; di Palumbo, A.S.; Sacchetti, M. Neuromuscular and metabolic responses to high-intensity intermittent cycling protocols with different work-to-rest ratios. Int. J. Sports Physiol. Perform. 2014, 9, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Puente-Maestu, L.; de Pedro, J.G.; Martínez-Abad, Y.; de Oña, J.M.R.; Llorente, D.; Cubillo, J.M. Dyspnea, ventilatory pattern, and changes in dynamic hyperinflation related to the intensity of constant work rate exercise in COPD. Chest 2005, 128, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, P.; Bussières, J.S.; Ribeiro, F.; Gagnon, S.L.; Saey, D.; Gagné, N.; Provencher, S.; Maltais, F. Influences of spinal anesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2012, 186, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Joint Committee for Guides in Metrology (JCGM). 200: 2012—International Vocabulary of Metrology—Basic and General Concepts and Associated Terms (VIM); Technical Report; JCGM: Sèvres, France, 2012. [Google Scholar]
- Rodríguez-Molinero, A.; Narvaiza, L.; Ruiz, J.; Gálvez-Barrón, C. Normal respiratory rate and peripheral blood oxygen saturation in the elderly population. J. Am. Geriatr. Soc. 2013, 61, 2238–2240. [Google Scholar] [CrossRef]
- Martin, E.A. Concise Medical Dictionary; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Ganong, W.F.; Ganong, W. Review of Medical Physiology; Appleton & Lange: Norwalk, CT, USA, 1995. [Google Scholar]
- Medical Technology. Available online: https://www.sensirion.com/en/markets/sensor-solutions-for-medical-applications/ (accessed on 12 December 2018).
- MIR Reusable Turbine. Available online: https://www.spirometry.com/Eng/Products/reusable_turbine.asp (accessed on 1 January 2019).
- Balakrishnan, V.; Phan, H.P.; Dinh, T.; Dao, D.V.; Nguyen, N.T. Thermal flow sensors for harsh environments. Sensors 2017, 17, 2061. [Google Scholar] [CrossRef]
- Schena, E.; Massaroni, C.; Saccomandi, P.; Cecchini, S. Flow measurement in mechanical ventilation: A review. Med. Eng. Phys. 2015, 37, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Stocks, J.; Sly, P.D.; Tepper, R.S.; Morgan, W.J. Infant Respiratory Function Testing; John Wiley & Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Fleisch, A. Der Pneumotachograph; ein Apparat zur Geschwindigkeitsregistrierung der Atemluft. Pflüegers Arch. Gesame Physiol. Menschen Tiere 1925, 209, 713–722. [Google Scholar] [CrossRef]
- Lilly, J.C. Flow meter for recording respiratory flow of human subjects. Methods Med. Res. 1950, 11, 113–121. [Google Scholar]
- Schena, E.; Lupi, G.; Cecchini, S.; Silvestri, S. Linearity dependence on oxygen fraction and gas temperature of a novel Fleisch pneumotachograph for neonatal ventilation at low flow rates. Measurement 2012, 45, 2064–2071. [Google Scholar] [CrossRef]
- Finucane, K.E.; Egan, B.A.; Dawson, S.V. Linearity and frequency response of pneumotachographs. J. Appl. Physiol. 1972, 32, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.W. Flow Measurement Engineering Handbook; McGraw-Hill: Columbus, OH, USA, 1983. [Google Scholar]
- Tardi, G.; Massaroni, C.; Saccomandi, P.; Schena, E. Experimental assessment of a variable orifice flowmeter for respiratory monitoring. J. Sens. 2015, 2015, 752540. [Google Scholar] [CrossRef]
- Stick, S.; Ellis, E.; LeSouëf, P.; Sly, P. Validation of respiratory inductance plethysmography (“Respitrace”®) for the measurement of tidal breathing parameters in newborns. Pediatr. Pulmonol. 1992, 14, 187–191. [Google Scholar] [CrossRef]
- Sharp, C.; Soleimani, V.; Hannuna, S.; Camplani, M.; Damen, D.; Viner, J.; Mirmehdi, M.; Dodd, J.W. Toward respiratory assessment using depth measurements from a time-of-flight sensor. Front. Physiol. 2017, 8, 65. [Google Scholar] [CrossRef]
- Presti, D.L.; Massaroni, C.; Formica, D.; Saccomandi, P.; Giurazza, F.; Caponero, M.A.; Schena, E. Smart Textile Based on 12 Fiber Bragg Gratings Array for Vital Signs Monitoring. IEEE Sens. J. 2017, 17, 6037–6043. [Google Scholar] [CrossRef]
- Beckwith, T.G.; Buck, N.L.; Marangoni, R.D. Mechanical Measurements; Addison-Wesley: Reading, MA, USA, 1969; Volume 5. [Google Scholar]
- Sokol, Y.; Tomashevsky, R.; Kolisnyk, K. Turbine spirometers metrological support. In Proceedings of the 2016 International Conference on Electronics and Information Technology (EIT), Odessa, Ukraine, 23–27 May 2016; pp. 1–4. [Google Scholar]
- Moore, V. Spirometry: Step by step. Breathe 2012, 8, 232–240. [Google Scholar] [CrossRef]
- Malmberg, L.; Hedman, J.; Sovijärvi, A. Accuracy and repeatability of a pocket turbine spirometer: Comparison with a rolling seal flow-volume spirometer. Clin. Physiol. 1993, 13, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Lucia, A.; Fleck, S.; Gotshall, R.; Kearney, J. Validity and reliability of the Cosmed K2 instrument. Int. J. Sports Med. 1993, 14, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Guidetti, L.; Meucci, M.; Bolletta, F.; Emerenziani, G.P.; Gallotta, M.C.; Baldari, C. Validity, reliability and minimum detectable change of COSMED K5 portable gas exchange system in breath-by-breath mode. PLoS ONE 2018, 13, e0209925. [Google Scholar] [CrossRef] [PubMed]
- Perez-Suarez, I.; Martin-Rincon, M.; Gonzalez-Henriquez, J.J.; Fezzardi, C.; Perez-Regalado, S.; Galvan-Alvarez, V.; Juan-Habib, J.W.; Morales-Alamo, D.; Calbet, J.A. Accuracy and precision of the COSMED K5 portable analyser. Front. Physiol. 2018, 9, 1764. [Google Scholar] [CrossRef] [PubMed]
- Bruun, H.H. Hot-wire Anemometry: Principles and Signal Analysis; Oxford Unversity Press: New York, NY, USA, 1996. [Google Scholar]
- King, L.V. XII. On the convection of heat from small cylinders in a stream of fluid: Determination of the convection constants of small platinum wires with applications to hot-wire anemometry. Philos. Trans. R. Soc. Lond. A 1914, 214, 373–432. [Google Scholar] [CrossRef]
- Yoshiya, I.; Nakajima, T.; Nagai, I.; Jitsukawa, S. A bidirectional respiratory flowmeter using the hot-wire principle. J. Appl. Physiol. 1975, 38, 360–365. [Google Scholar] [CrossRef]
- Te Pas, A.B.; Wong, C.; Kamlin, C.O.F.; Dawson, J.A.; Morley, C.J.; Davis, P.G. Breathing patterns in preterm and term infants immediately after birth. Pediatr. Res. 2009, 65, 352. [Google Scholar] [CrossRef]
- Hager, D.N.; Fuld, M.; Kaczka, D.W.; Fessler, H.E.; Brower, R.G.; Simon, B.A. Four methods of measuring tidal volume during high-frequency oscillatory ventilation. Crit. Care Med. 2006, 34, 751–757. [Google Scholar] [CrossRef]
- Sturtz, W.J.; Touch, S.M.; Locke, R.G.; Greenspan, J.S.; Shaffer, T.H. Assessment of neonatal ventilation during high-frequency oscillatory ventilation. Pediatr. Crit. Care Med. 2008, 9, 101–104. [Google Scholar] [CrossRef]
- Shikida, M.; Naito, J.; Yokota, T.; Kawabe, T.; Hayashi, Y.; Sato, K. A catheter-type flow sensor for measurement of aspirated-and inspired-air characteristics in the bronchial region. J. Micromech. Microeng. 2009, 19, 105027. [Google Scholar] [CrossRef]
- Jiang, P.; Zhao, S.; Zhu, R. Smart sensing strip using monolithically integrated flexible flow sensor for noninvasively monitoring respiratory flow. Sensors 2015, 15, 31738–31750. [Google Scholar] [CrossRef] [PubMed]
- Schena, E.; Saccomandi, P.; Silvestri, S. A high sensitivity fiber optic macro-bend based gas flow rate transducer for low flow rates: Theory, working principle, and static calibration. Rev. Sci. Instrum. 2013, 84, 024301. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.P.; Huang, X.G. A simple fiber-optic flowmeter based on bending loss. IEEE Sens. J. 2009, 9, 1952–1955. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, K.; Yang, J. Novel target type flowmeter based on a differential fiber Bragg grating sensor. Measurement 2005, 38, 230–235. [Google Scholar] [CrossRef]
- Lu, P.; Chen, Q. Fiber Bragg grating sensor for simultaneous measurement of flow rate and direction. Meas. Sci. Technol. 2008, 19, 125302. [Google Scholar] [CrossRef]
- Lim, J.; Yang, Q.; Jones, B.; Jackson, P. DP flow sensor using optical fibre Bragg grating. Sens. Actuators A Phys. 2001, 92, 102–108. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, A.P.; Tam, H.Y.; Cho, L.; Lu, C. All-optical fiber anemometer based on laser heated fiber Bragg gratings. Opt. Express 2011, 19, 10124–10130. [Google Scholar] [CrossRef]
- Mohanty, L.; Kuang, K.S. A breathing rate sensor with plastic optical fiber. Appl. Phys. Lett. 2010, 97, 073703. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Kumar, R.; Ng, W.P.; Fu, Y.Q.; Yuan, J.; Yu, C.; Wu, Y.; Zhou, G.; Farrell, G.; et al. A simple optical fiber interferometer based breathing sensor. Meas. Sci. Technol. 2017, 28, 035105. [Google Scholar] [CrossRef] [Green Version]
- Battista, L.; Sciuto, S.; Scorza, A. An air flow sensor for neonatal mechanical ventilation applications based on a novel fiber-optic sensing technique. Rev. Sci. Instrum. 2013, 84, 035005. [Google Scholar] [CrossRef]
- Saccomandi, P.; Schena, E.; Silvestri, S. A novel target-type low pressure drop bidirectional optoelectronic air flow sensor for infant artificial ventilation: Measurement principle and static calibration. Rev. Sci. Instrum. 2011, 82, 024301. [Google Scholar] [CrossRef] [PubMed]
- Sovijarvi, A.; Dalmasso, F.; Vanderschoot, J.; Malmberg, L.; Righini, G.; Stoneman, S. Definition of terms for applications of respiratory sounds. Eur. Respir. Rev. 2000, 10, 597–610. [Google Scholar]
- Ballantine, D., Jr.; White, R.M.; Martin, S.J.; Ricco, A.J.; Zellers, E.; Frye, G.; Wohltjen, H. Acoustic Wave Sensors: Theory, Design and Physico-Chemical Applications; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Li, S.H.; Lin, B.S.; Tsai, C.H.; Yang, C.T.; Lin, B.S. Design of wearable breathing sound monitoring system for real-time wheeze detection. Sensors 2017, 17, 171. [Google Scholar] [CrossRef]
- Eargle, J. The Microphone Book: From Mono to Stereo to Surround—A Guide To Microphone Design and Application; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Eargle, J.M. Handbook of Recording Engineering; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Moussavi, Z. Fundamentals of respiratory sounds and analysis. Synth. Lect. Biomed. Eng. 2006, 1, 1–68. [Google Scholar] [CrossRef]
- Reyes, B.A.; Reljin, N.; Chon, K.H. Tracheal sounds acquisition using smartphones. Sensors 2014, 14, 13830–13850. [Google Scholar] [CrossRef] [PubMed]
- Reichert, S.; Gass, R.; Brandt, C.; Andrès, E. Analysis of respiratory sounds: State of the art. Clin. Med. Circ. Respir. Pulm. Med. 2008, 2, 45–58. [Google Scholar] [CrossRef]
- Mimoz, O.; Benard, T.; Gaucher, A.; Frasca, D.; Debaene, B. Accuracy of respiratory rate monitoring using a non-invasive acoustic method after general anaesthesia. Br. J. Anaesth. 2012. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 327, 307–310. [Google Scholar] [CrossRef]
- Sierra, G.; Telfort, V.; Popov, B.; Pelletier, M.; Despault, P.; Lanzo, V.; Agarwal, R. Comparison of respiratory rate estimation based on tracheal sounds versus a capnograph. In Proceedings of the 27th Annual International Conference of the Engineering in Medicine and Biology Society (IEEE-EMBS 2005), Shanghai, China, 17–18 January 2006; pp. 6145–6148. [Google Scholar]
- Corbishley, P.; Rodríguez-Villegas, E. Breathing detection: Towards a miniaturized, wearable, battery-operated monitoring system. IEEE Trans. Biomed. Eng. 2008. [Google Scholar] [CrossRef]
- Gu, F.; Niu, J.; Das, S.K.; He, Z.; Jin, X. Detecting breathing frequency and maintaining a proper running rhythm. Pervasive Mob. Comput. 2017, 42, 498–512. [Google Scholar] [CrossRef]
- Oletic, D.; Bilas, V. Energy-efficient respiratory sounds sensing for personal mobile asthma monitoring. IEEE Sens. J. 2016, 16, 8295–8303. [Google Scholar] [CrossRef]
- Wang, Y.D.; Liu, C.H.; Jiang, R.Y.; Lin, B.S.; Lin, B.S. Novel Approach of Respiratory Sound Monitoring under Motion. In International Conference on Intelligent Information Hiding and Multimedia Signal Processing; Springer: Berlin, Germany, 2017; pp. 167–174. [Google Scholar]
- Höppe, P. Temperatures of expired air under varying climatic conditions. Int. J. Biometeorol. 1981, 25, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.C.; Siao, A.S.; Ciou, J.C. Improvement of pyroelectric cells for thermal energy harvesting. Sensors 2012, 12, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Kim, C.M.; Choi, S.Y.; Lee, B.Y. Enhanced strain measurement range of an FBG sensor embedded in seven-wire steel strands. Sensors 2017, 17, 1654. [Google Scholar] [CrossRef] [PubMed]
- Storck, K.; Karlsson, M.; Ask, P.; Loyd, D. Heat transfer evaluation of the nasal thermistor technique. IEEE Trans. Biomed. Eng. 1996, 43, 1187–1191. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Young, M.S.; Tai, C. Noninvasive respiratory monitoring system based on the piezoceramic transducer’s pyroelectric effect. Rev. Sci. Instrum. 2008, 79, 035103. [Google Scholar] [CrossRef] [PubMed]
- Carskadon, M.A.; Harvey, K.; Dement, W.C.; Guilleminault, C.; Simmons, F.B.; Anders, T.F. Respiration during sleep in children. West. J. Med. 1978, 128, 477. [Google Scholar]
- Suzuki, S.; Matsui, T.; Kawahara, H.; Ichiki, H.; Shimizu, J.; Kondo, Y.; Gotoh, S.; Yura, H.; Takase, B.; Ishihara, M. A non-contact vital sign monitoring system for ambulances using dual-frequency microwave radars. Med. Biol. Eng. Comput. 2009, 47, 101–105. [Google Scholar] [CrossRef]
- Ţarălungă, D.D.; Mocanu, B.; Ţapu, R. Automatic real time derivation of breathing rate from thermal video sequences. IFMBE Proc. 2017. [Google Scholar] [CrossRef]
- Van Herwaarden, A.W.; Sarro, P.M. Thermal sensors based on the seebeck effect. Sens. Actuators 1986. [Google Scholar] [CrossRef]
- Burns, G.; Scroger, M. The Calibration of Thermocouples and Thermocouple Materials. Natl. Inst. Stand. Technol. 1989. [Google Scholar] [CrossRef]
- Marks, M.K.; South, M.; Carter, B.G. Measurement of respiratory rate and timing using a nasal thermocouple. J. Clin. Monit. 1995. [Google Scholar] [CrossRef]
- Lim, S.; Park, S.H.; Do Ahn, S.; Suh, Y.; Shin, S.S.; Lee, S.W.; Kim, J.H.; Choi, E.K.; Yi, B.Y.; Kwon, S.I.; et al. Guiding curve based on the normal breathing as monitored by thermocouple for regular breathing. Med. Phys. 2007. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.T.; Lim, S.; Kwon, S.I.; Kim, C.M.; Park, S.H.; Shin, S.S.; Lee, S.; Ahn, S.D.; Kim, J.H.; Choi, E.K. Comparison of thermocouple, spirometer and skin motion for respiratory target motion measurement. In World Congress on Medical Physics and Biomedical Engineering 2006; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1977–1979. [Google Scholar] [CrossRef]
- Itasaka, Y.; Miyazaki, S.; Tanaka, T.; Shibata, Y.; Ishikawa, K. Detection of Respiratory Events during Polysomnography: Nasal-Oral Pressure Sensor Versus Thermocouple Airflow Sensor. Pract. Oto-Rhino-Laryngol. Suppl. 2010, 129, 60–63. [Google Scholar] [CrossRef]
- Lim, S.; Park, S.; Ahn, S.; Yi, B.; Shin, S.; Lee, S.; Kim, J.; Choi, E.; Kwon, S.; Jeung, T. SU-FF-J-41: Comparison of Various Respiration Measurement Methods for 4D Radiotherapy. Med. Phys. 2006, 33, 2029. [Google Scholar] [CrossRef]
- Thomson, W., II. On the thermoelastic, thermomagnetic, and pyroelectric properties of matter. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1878, 5, 4–27. [Google Scholar] [CrossRef]
- Webster, J.G. The Measurement, Instrumentation and Sensors Handbook; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Cooper, J. A fast-response pyroelectric thermal detector. J. Sci. Instrum. 1962, 39, 467. [Google Scholar] [CrossRef]
- Huang, Y.P.; Huang, K.N. Monitoring of breathing rate by a piezofilm sensor using pyroelectric effect. In Proceedings of the 2013 International Conference on Orange Technologies (ICOT), Tainan, Taiwan, 12–16 March 2013; pp. 99–102. [Google Scholar]
- Krohn, D.A.; MacDougall, T.; Mendez, A. Fiber Optic Sensors: Fundamentals and Applications; Spie Press: Bellingham, WA, USA, 2014. [Google Scholar]
- Schena, E.; Tosi, D.; Saccomandi, P.; Lewis, E.; Kim, T. Fiber optic sensors for temperature monitoring during thermal treatments: An overview. Sensors 2016, 16, 1144. [Google Scholar] [CrossRef]
- Liang, Y.; Mazzolini, A.P.; Stoddart, P.R. Fibre Bragg grating sensor for respiratory monitoring. In Proceedings of the ACOFT/AOS 2006—Australian Conference on Optical Fibre Technology/Australian Optical Society, Melbourne, Australia, 10–13 July 2006. [Google Scholar]
- Yoo, W.J.; Jang, K.W.; Seo, J.K.; Heo, J.Y.; Moon, J.S.; Park, J.Y.; Lee, B.S. Development of respiration sensors using plastic optical fiber for respiratory monitoring inside MRI system. J. Opt. Soc. Korea 2010, 14, 235–239. [Google Scholar] [CrossRef]
- Massaroni, C.; Presti, D.L.; Saccomandi, P.; Caponero, M.A.; D’Amato, R.; Schena, E. Fiber Bragg Grating Probe for Relative Humidity and Respiratory Frequency Estimation: Assessment During Mechanical Ventilation. IEEE Sens. J. 2018, 18, 2125–2130. [Google Scholar] [CrossRef]
- Branson, R.D.; Gentile, M.A. Is humidification always necessary during noninvasive ventilation in the hospital? Respir. Care 2010, 55, 209–216. [Google Scholar] [PubMed]
- Lee, S.P. Synthesis and characterization of carbon nitride films for micro humidity sensors. Sensors 2008, 8, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, C.; Olkkonen, J.; Passoja, S.; Smolander, M. Paper as active layer in inkjet-printed capacitive humidity sensors. Sensors 2017, 17, 1464. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, J.; Ding, C.; Bai, G.; Xu, J.; Ren, Q.; Li, J. Fabrication of Ordered SnO2 Nanostructures with Enhanced Humidity Sensing Performance. Sensors 2017, 17, 2392. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Principe, S.; Consales, M.; Parente, R.; Laudati, A.; Caliro, S.; Cutolo, A.; Cusano, A. Fiber Optic Thermo-Hygrometers for Soil Moisture Monitoring. Sensors 2017, 17, 1451. [Google Scholar] [CrossRef] [PubMed]
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle, mechanism, and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef]
- Rittersma, Z. Recent achievements in miniaturised humidity sensors—A review of transduction techniques. Sens. Actuators A Phys. 2002, 96, 196–210. [Google Scholar] [CrossRef]
- Tatara, T.; Tsuzaki, K. An apnea monitor using a rapid-response hygrometer. J. Clin. Monit. 1997, 13, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, A.K.; Li, H.; O’Brien, C.J.; Fonash, S.J. A rapid-response, high-sensitivity nanophase humidity sensor for respiratory monitoring. IEEE Electron Device Lett. 2004. [Google Scholar] [CrossRef]
- Kupsta, M.R.; Taschuk, M.T.; Brett, M.J.; Sit, J.C. Reactive Ion Etching of Columnar Nanostructured TiO2 Thin Films for Modified Relative Humidity Sensor Response Time. IEEE Sens. J. 2009. [Google Scholar] [CrossRef]
- Tetelin, A.; Pellet, C.; Achen, A.; Toepper, M. Capacitive humidity sensors based on oxidized PhotoBCB polymer films: Enhanced sensitivity and response time. In Proceedings of the 2005 IEEE SENSORS, Irvine, CA, USA, 30 October–3 November 2005. [Google Scholar]
- Scholz, R.; Bracio, B.R.; Brutscheck, M.; Trommler, P. Non-invasive respiratory rate detection in spontaneous respiration by humidity measurement. In Proceedings of the 2017 28th Irish Signals and Systems Conference (ISSC), Killarney, Ireland, 20–21 June 2017; pp. 1–6. [Google Scholar]
- Niesters, M.; Mahajan, R.; Olofsen, E.; Boom, M.; Garcia Del Valle, S.; Aarts, L.; Dahan, A. Validation of a novel respiratory rate monitor based on exhaled humidity. Br. J. Anaesth. 2012. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhang, L.; Yu, P.; Mao, L. Sensitive and Fast Humidity Sensor Based on A Redox Conducting Supramolecular Ionic Material for Respiration Monitoring. Anal. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kano, S.; Dobashi, Y.; Fujii, M. Silica Nanoparticle-Based Portable Respiration Sensor for Analysis of Respiration Rate, Pattern, and Phase During Exercise. IEEE Sens. Lett. 2018, 2, 1–4. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Sun, Y.E.; Li, P.; Zhang, Y. Facile Fabrication of MoS2-Modified SnO2 Hybrid Nanocomposite for Ultrasensitive Humidity Sensing. Acs Appl. Mater. Interfaces 2016. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Kim, H.K.; Kim, T.; Bae, K.M.; Seo, S.M.; Kim, J.M.; Kang, T.J.; Kim, Y.H. Self-powered humidity sensor based on graphene oxide composite film intercalated by poly (sodium 4-styrenesulfonate). ACS Appl. Mater. Interfaces 2014, 6, 8320–8326. [Google Scholar] [CrossRef] [PubMed]
- Kano, S.; Kim, K.; Fujii, M. Fast-response and flexible nanocrystal-based humidity sensor for monitoring human respiration and water evaporation on skin. ACS Sens. 2017, 2, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Güder, F.; Ainla, A.; Redston, J.; Mosadegh, B.; Glavan, A.; Martin, T.; Whitesides, G.M. Paper-based electrical respiration sensor. Angew. Chem. Int. Ed. 2016, 55, 5727–5732. [Google Scholar] [CrossRef]
- Yeo, T.L.; Sun, T.; Grattan, K.T. Fibre-optic sensor technologies for humidity and moisture measurement. Sens. Actuators A Phys. 2008, 144, 280–295. [Google Scholar] [CrossRef]
- Ascorbe, J.; Corres, J.; Arregui, F.; Matias, I. Recent Developments in Fiber Optics Humidity Sensors. Sensors 2017, 17, 893. [Google Scholar] [CrossRef]
- Presti, D.L.; Massaroni, C.; Schena, E. Optical Fiber Gratings for Humidity Measurements: A Review. IEEE Sens. J. 2018, 18, 9065–9074. [Google Scholar] [CrossRef]
- Presti, D.L.; Massaroni, C.; Piemonte, V.; Saccomandi, P.; D’Amato, R.; Caponero, M.; Schena, E. Agar-coated fiber Bragg grating sensor for relative humidity measurements: Influence of coating thickness and polymer concentration. IEEE Sens. J. 2019. [Google Scholar] [CrossRef]
- Shivananju, B.N.; Yamdagni, S.; Fazuldeen, R.; Kumar, A.K.S.; Nithin, S.P.; Varma, M.M.; Asokan, S. Highly sensitive carbon nanotubes coated etched fiber bragg grating sensor for humidity sensing. IEEE Sens. J. 2014. [Google Scholar] [CrossRef]
- Viegas, D.; Goicoechea, J.; Corres, J.M.; Santos, J.L.; Ferreira, L.A.; Arajo, F.M.; Matias, I.R. A fibre optic humidity sensor based on a long-period fibre grating coated with a thin film of SiO2 nanospheres. Meas. Sci. Technol. 2009. [Google Scholar] [CrossRef]
- Corres, J.M.; Matias, I.R.; Hernaez, M.; Bravo, J.; Arregui, F.J. Optical Fiber Humidity Sensors Using Nanostructured Coatings of SiO2 Nanoparticles. IEEE Sens. J. 2008. [Google Scholar] [CrossRef]
- Mathew, J.; Semenova, Y.; Farrell, G. Relative Humidity Sensor Based on an Agarose-Infiltrated Photonic Crystal Fiber Interferometer. IEEE J. Sel. Top. Quantum Electron. 2012. [Google Scholar] [CrossRef]
- Zhang, W.; Webb, D.J.; Peng, G.D. Investigation into time response of polymer fiber bragg grating based humidity sensors. J. Lightwave Technol. 2012. [Google Scholar] [CrossRef]
- Yan, G.; Liang, Y.; Lee, E.H.; He, S. Novel Knob-integrated fiber Bragg grating sensor with polyvinyl alcohol coating for simultaneous relative humidity and temperature measurement. Opt. Express 2015. [Google Scholar] [CrossRef] [PubMed]
- Mathew, J.; Semenova, Y.; Farrell, G. A miniature optical humidity sensor. In Proceedings of the Sensors, 2011 IEEE, Limerick, Ireland, 28–31 October 2011; pp. 2030–2033. [Google Scholar]
- Hernandez, F.; Correia, R.; Morgan, S.; Hayes-Gill, B.; Evans, D.; Sinha, R.; Norris, A.; Harvey, D.; Hardman, J.; Korposh, S. Simultaneous temperature and humidity measurements in a mechanical ventilator using an optical fibre sensor. Int. Soc. Opt. Photonics 2016, 9916, 99160C. [Google Scholar] [Green Version]
- Massaroni, C.; Presti, D.L.; Losquadro, C.; Resta, P.; Saccomandi, P.; Schena, E.; D’Amato, R.; Caponero, M.A. Multi-sensitive FBG-based needle for both relative humidity and breathing rate monitoring. In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018; pp. 1–6. [Google Scholar]
- Massaroni, C.; Caponero, M.A.; D’Amato, R.; Lo Presti, D.; Schena, E. Fiber Bragg grating measuring system for simultaneous monitoring of temperature and humidity in mechanical ventilation. Sensors 2017, 17, 749. [Google Scholar] [CrossRef]
- Iacoponi, S.; Massaroni, C.; Presti, D.L.; Saccomandi, P.; Caponero, M.; D’Amato, R.; Schena, E. Polymer-coated fiber optic probe for the monitoring of breathing pattern and respiratory rate. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 1616–1619. [Google Scholar]
- Vegfors, M.; Lindberg, L.G.; Pettersson, H.; Öberg, P.Å. Presentation and evaluation of a new optical sensor for respiratory rate monitoring. Int. J. Clin. Monit. Comput. 1994, 11, 151–156. [Google Scholar] [CrossRef]
- Nurulain, S.; Radin, M.; Suzalina, K.; Manap, H. Spectra comparison for an optical breathing gas sensor development. AIP Conf. Proc. 2017, 1835, 020035. [Google Scholar] [Green Version]
- Singh, O.P.; Howe, T.A.; Malarvili, M. Real-time human respiration carbon dioxide measurement device for cardiorespiratory assessment. J. Breath Res. 2018, 12, 026003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katagiri, T.; Shibayama, K.; Iida, T.; Matsuura, Y. Infrared Hollow Optical Fiber Probe for Localized Carbon Dioxide Measurement in Respiratory Tracts. Sensors 2018, 18, 995. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.V.; Choi, I.Y.; Son, Y.S.; Kim, J.C. A review on non-dispersive infrared gas sensors: Improvement of sensor detection limit and interference correction. Sens. Actuators B Chem. 2016, 231, 529–538. [Google Scholar] [CrossRef]
- Jaffe, M.B. Infrared measurement of carbon dioxide in the human breath: “Breathe-through” devices from Tyndall to the present day. Anesth. Analg. 2008, 107, 890–904. [Google Scholar] [CrossRef] [PubMed]
- Accurate Capnography Highly Dependent on a Quality Sampling Line. Available online: https://www.medtronic.com/content/dam/covidien/library/us/en/product/capnography-monitoring/capnography-quality-sampling-lines-white-paper.pdf (accessed on 9 December 2018).
- Yang, J.; Chen, B.; Zhou, J.; Lv, Z. A low-power and portable biomedical device for respiratory monitoring with a stable power source. Sensors 2015, 15, 19618–19632. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Chiang, C.C. Notched long-period fiber grating with an amine-modified surface nanostructure for carbon dioxide gas sensing. Materials 2015, 8, 4535–4543. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Chiang, C.C. Sandwiched long-period fiber grating fabricated by MEMS process for CO2 gas detection. Micromachines 2016, 7, 35. [Google Scholar] [CrossRef]
- Ma, W.; Xing, J.; Wang, R.; Rong, Q.; Zhang, W.; Li, Y.; Zhang, J.; Qiao, X. Optical fiber Fabry–Perot interferometric CO2 gas sensor using guanidine derivative polymer functionalized layer. IEEE Sens. J. 2018, 18, 1924–1929. [Google Scholar] [CrossRef]
- Moll, J.M.; Wright, V. An objective clinical study of chest expansion. Ann. Rheum. Dis. 1972. [Google Scholar] [CrossRef]
- Fiorillo, A.; Critello, C.; Pullano, A. Theory, technology and applications of piezoresistive sensors: A review. Sens. Actuators A Phys. 2018. [Google Scholar] [CrossRef]
- Kim, K.; Song, G.; Park, C.; Yun, K.S. Multifunctional Woven Structure Operating as Triboelectric Energy Harvester, Capacitive Tactile Sensor Array, and Piezoresistive Strain Sensor Array. Sensors 2017, 17, 2582. [Google Scholar] [CrossRef] [PubMed]
- Terazawa, M.; Karita, M.; Kumagai, S.; Sasaki, M. Respiratory Motion Sensor Measuring Capacitance Constructed across Skin in Daily Activities. Micromachines 2018, 9, 543. [Google Scholar] [CrossRef] [PubMed]
- Fajkus, M.; Nedoma, J.; Martinek, R.; Vasinek, V.; Nazeran, H.; Siska, P. A non-invasive multichannel hybrid fiber-optic sensor system for vital sign monitoring. Sensors 2017, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, D.; Carpi, F.; Lorussi, F.; Mazzoldi, A.; Paradiso, R.; Scilingo, E.P.; Tognetti, A. Electroactive fabrics and wearable biomonitoring devices. AUTEX Res. J. 2003, 3, 180–185. [Google Scholar]
- Wang, J.; Xue, P.; Tao, X. Strain sensing behavior of electrically conductive fibers under large deformation. Mater. Sci. Eng. A 2011, 528, 2863–2869. [Google Scholar] [CrossRef]
- Egami, Y.; Suzuki, K.; Tanaka, T.; Yasuhara, T.; Higuchi, E.; Inoue, H. Preparation and characterization of conductive fabrics coated uniformly with polypyrrole nanoparticles. Synth. Met. 2011, 161, 219–224. [Google Scholar] [CrossRef]
- Huang, C.T.; Shen, C.L.; Tang, C.F.; Chang, S.H. A wearable yarn-based piezo-resistive sensor. Sens. Actuators A Phys. 2008, 141, 396–403. [Google Scholar] [CrossRef]
- Atalay, O.; Kennon, W.R.; Demirok, E. Weft-knitted strain sensor for monitoring respiratory rate and its electro-mechanical modeling. IEEE Sens. J. 2015, 15, 110–122. [Google Scholar] [CrossRef]
- Lanatà, A.; Scilingo, E.P.; Nardini, E.; Loriga, G.; Paradiso, R.; De-Rossi, D. Comparative evaluation of susceptibility to motion artifact in different wearable systems for monitoring respiratory rate. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 378–386. [Google Scholar] [CrossRef]
- Paradiso, R.; Loriga, G.; Taccini, N. A wearable health care system based on knitted integrated sensors. IEEE Trans. Inf. Technol. Biomed. 2005, 9, 337–344. [Google Scholar] [CrossRef]
- Hamdani, S.T.A.; Fernando, A. The application of a piezo-resistive cardiorespiratory sensor system in an automobile safety belt. Sensors 2015, 15, 7742–7753. [Google Scholar] [CrossRef]
- Jeong, J.; Jang, Y.; Lee, I.; Shin, S.; Kim, S. Wearable respiratory rate monitoring using piezo-resistive fabric sensor. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Munich, Germany, 7–12 September 2009; Springer: Berlin, Germany, 2009; pp. 282–284. [Google Scholar]
- Molinaro, N.; Massaroni, C.; Presti, D.L.; Saccomandi, P.; Di Tomaso, G.; Zollo, L.; Perego, P.; Andreoni, G.; Schena, E. Wearable textile based on silver plated knitted sensor for respiratory rate monitoring. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 2865–2868. [Google Scholar]
- Kundu, S.K.; Kumagai, S.; Sasaki, M. A wearable capacitive sensor for monitoring human respiratory rate. Jpn. J. Appl. Phys. 2013, 52, 04CL05. [Google Scholar] [CrossRef]
- Grlica, J.; Martinović, T.; Džapo, H. Capacitive sensor for respiration monitoring. In Proceedings of the 2015 IEEE Sensors Applications Symposium (SAS), Zadar, Croatia, 13–15 April 2015; pp. 1–6. [Google Scholar]
- Ghasemzadeh, H.; Ostadabbas, S.; Guenterberg, E.; Pantelopoulos, A. Wireless medical-embedded systems: A review of signal-processing techniques for classification. IEEE Sens. J. 2013, 13, 423–437. [Google Scholar] [CrossRef]
- Takano, M.; Yamagishi, S.; Ohmuta, T.; Fukuoka, Y.; Ueno, A. Non-contact simultaneous measurements of electrocardiogram and respiratory movements using capacitive sheet electrodes. Adv. Biomed. Eng. 2017, 6, 28–36. [Google Scholar] [CrossRef]
- Luis, J.A.; Roa Romero, L.M.; Gómez-Galán, J.A.; Hernández, D.N.; Estudillo-Valderrama, M.Á.; Barbarov-Rostán, G.; Rubia-Marcos, C. Design and implementation of a smart sensor for respiratory rate monitoring. Sensors 2014, 14, 3019–3032. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, D.; Foussier, J.; Jia, J.; Leonhardt, S. Non-contacting monitoring of respiration and pulse based on capacitive coupling with thoracic tissue. Proc. World Congr. Eng. 2011, 3, 2695–2698. [Google Scholar]
- Naranjo-Hernández, D.; Talaminos-Barroso, A.; Reina-Tosina, J.; Roa, L.; Barbarov-Rostan, G.; Cejudo-Ramos, P.; Márquez-Martín, E.; Ortega-Ruiz, F. Smart Vest for Respiratory Rate Monitoring of COPD Patients Based on Non-Contact Capacitive Sensing. Sensors 2018, 18, 2144. [Google Scholar] [CrossRef] [PubMed]
- Chadha, T.; Watson, H.; Birch, S.; Jenouri, G.; Schneider, A.; Cohn, M.; Sackner, M. Validation of respiratory inductive plethysmography using different calibration procedures. Am. Rev. Respir. Dis. 1982, 125, 644–649. [Google Scholar]
- Dall’Ava-Santucci, J.; Armanganidis, A. Respiratory inductive plethysmography. In Pulmonary Function in Mechanically Ventilated Patients; Springer: Berlin, Germany, 1991; pp. 121–142. [Google Scholar]
- Krieger, B.; Feinerman, D.; Zaron, A.; Bizousky, F. Continuous noninvasive monitoring of respiratory rate in critically III patients. Chest 1986, 90, 632–634. [Google Scholar] [CrossRef]
- Mayer, O.H.; Clayton, R.G., Sr.; Jawad, A.F.; McDonough, J.M.; Allen, J.L. Respiratory inductance plethysmography in healthy 3- to 5-year-old children. Chest 2003, 124, 1812–1819. [Google Scholar] [CrossRef]
- Cantineau, J.P.; Escourrou, P.; Sartene, R.; Gaultier, C.; Goldman, M. Accuracy of respiratory inductive plethysmography during wakefulness and sleep in patients with obstructive sleep apnea. Chest 1992, 102, 1145–1151. [Google Scholar] [CrossRef]
- Fiamma, M.N.; Samara, Z.; Baconnier, P.; Similowski, T.; Straus, C. Respiratory inductive plethysmography to assess respiratory variability and complexity in humans. Respir. Physiol. Neurobiol. 2007, 156, 234–239. [Google Scholar] [CrossRef]
- Caretti, D.M.; Pullen, P.V.; Premo, L.A.; Kuhlmann, W.D. Reliability of respiratory inductive plethysmography for measuring tidal volume during exercise. Am. Ind. Hyg. Assoc. J. 1994, 55, 918–923. [Google Scholar] [CrossRef]
- Massaroni, C.; Carraro, E.; Vianello, A.; Miccinilli, S.; Morrone, M.; Levai, I.K.; Schena, E.; Saccomandi, P.; Sterzi, S.; Dickinson, J.W.; et al. Optoelectronic plethysmography in clinical practice and research: A review. Respiration 2017, 93, 339–354. [Google Scholar] [CrossRef]
- Pereira, M.C.; Porras, D.C.; Lunardi, A.C.; da Silva, C.C.B.M.; Barbosa, R.C.C.; Cardenas, L.Z.; Pletsch, R.; Ferreira, J.G.; de Castro, I.; de Carvalho, C.R.F.; et al. Thoracoabdominal asynchrony: Two methods in healthy, COPD, and interstitial lung disease patients. PLoS ONE 2017, 12, e0182417. [Google Scholar] [CrossRef]
- Clarenbach, C.F.; Senn, O.; Brack, T.; Kohler, M.; Bloch, K.E. Monitoring of ventilation during exercise by a portable respiratory inductive plethysmograph. Chest 2005, 128, 1282–1290. [Google Scholar] [CrossRef]
- Massaroni, C.; Saccomandi, P.; Schena, E. Medical smart textiles based on fiber optic technology: An overview. J. Funct. Biomater. 2015, 6, 204–221. [Google Scholar] [CrossRef]
- Scherer, L.J.; Boesel, L.F.; Wolf, M.; Bona, G.L.; Rossi, R.M. Body-Monitoring and Health Supervision by Means of Optical Fiber-Based Sensing Systems in Medical Textiles. Adv. Healthc. Mater. 2015, 4, 330–355. [Google Scholar]
- Dziuda, L.; Skibniewski, F.W.; Krej, M.; Lewandowski, J. Monitoring respiration and cardiac activity using fiber Bragg grating-based sensor. IEEE Trans. Biomed. Eng. 2012, 59, 1934–1942. [Google Scholar] [CrossRef]
- Dziuda, L.; Krej, M.; Skibniewski, F.W. Fiber Bragg grating strain sensor incorporated to monitor patient vital signs during MRI. IEEE Sens. J. 2013, 13, 4986–4991. [Google Scholar] [CrossRef]
- Chethana, K.; Guru Prasad, A.; Omkar, S.; Asokan, S. Fiber bragg grating sensor based device for simultaneous measurement of respiratory and cardiac activities. J. Biophotonics 2017, 10, 278–285. [Google Scholar] [CrossRef]
- Ciocchetti, M.; Massaroni, C.; Saccomandi, P.; Caponero, M.A.; Polimadei, A.; Formica, D.; Schena, E. Smart textile based on fiber bragg grating sensors for respiratory monitoring: Design and preliminary trials. Biosensors 2015, 5, 602–615. [Google Scholar] [CrossRef]
- Massaroni, C.; Saccomandi, P.; Formica, D.; Presti, D.L.; Caponero, M.A.; Di Tomaso, G.; Giurazza, F.; Muto, M.; Schena, E. Design and feasibility assessment of a magnetic resonance-compatible smart textile based on fiber Bragg grating sensors for respiratory monitoring. IEEE Sens. J. 2016, 16, 8103–8110. [Google Scholar] [CrossRef]
- Massaroni, C.; Venanzi, C.; Silvatti, A.P.; Lo Presti, D.; Saccomandi, P.; Formica, D.; Giurazza, F.; Caponero, M.A.; Schena, E. Smart textile for respiratory monitoring and thoraco-abdominal motion pattern evaluation. J. Biophotonics 2018, 11, e201700263. [Google Scholar] [CrossRef]
- Lo Presti, D.; Massaroni, C.; Saccomandi, P.; Caponero, M.A.; Formica, D.; Schena, E. A wearable textile for respiratory monitoring: Feasibility assessment and analysis of sensors position on system response. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Seogwipo, South Korea, 11–15 July 2017; Volume 2017, pp. 4423–4426. [Google Scholar]
- Krehel, M.; Schmid, M.; Rossi, R.M.; Boesel, L.F.; Bona, G.L.; Scherer, L.J. An optical fibre-based sensor for respiratory monitoring. Sensors 2014, 14, 13088–13101. [Google Scholar] [CrossRef]
- Augousti, A.; Maletras, F.; Mason, J. Improved fibre optic respiratory monitoring using a figure-of-eight coil. Physiol. Meas. 2005, 26, 585–590. [Google Scholar] [CrossRef]
- Koyama, Y.; Nishiyama, M.; Watanabe, K. Smart textile using hetero-core optical fiber for heartbeat and respiration monitoring. IEEE Sens. J. 2018, 18, 6175–6180. [Google Scholar] [CrossRef]
- Lau, D.; Chen, Z.; Teo, J.T.; Ng, S.H.; Rumpel, H.; Lian, Y.; Yang, H.; Kei, P.L. Intensity-modulated microbend fiber optic sensor for respiratory monitoring and gating during MRI. IEEE Trans. Biomed. Eng. 2013, 60, 2655–2662. [Google Scholar] [CrossRef]
- Gupta, A.K. Respiration Rate Measurement Based on Impedance Pneumography; Application Report SBAA181; Texas Instruments: Dallas, TX, USA, 2011. [Google Scholar]
- Trobec, R.; Rashkovska, A.; Avbelj, V. Two proximal skin electrodes—A respiration rate body sensor. Sensors 2012, 12, 13813–13828. [Google Scholar] [CrossRef]
- Malmivuo, P.; Malmivuo, J.; Plonsey, R. Bioelectromagnetism: Principles and Applications of Bioelectric and Biomagnetic Fields; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Larsen, V.H.; Christensen, P.H.; Oxhøj, H.; Brask, T. Impedance pneumography for long-term monitoring of respiration during sleep in adult males. Clin. Physiol. 1984, 4, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Lee-Chiong, T.L. Monitoring respiration during sleep. Clin. Chest Med. 2003, 24, 297–306. [Google Scholar] [CrossRef]
- Wang, F.T.; Chan, H.L.; Wang, C.L.; Jian, H.M.; Lin, S.H. Instantaneous respiratory estimation from thoracic impedance by empirical mode decomposition. Sensors 2015, 15, 16372–16387. [Google Scholar] [CrossRef] [PubMed]
- Houtveen, J.H.; Groot, P.F.; de Geus, E.J. Validation of the thoracic impedance derived respiratory signal using multilevel analysis. Int. J. Psychophysiol. 2006, 59, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ravenswaaij-Arts, C.; Hopman, J.; Kollee, L.; Stoelinga, G.; Van Geijn, H. Spectral analysis of heart rate variability in spontaneously breathing very preterm infants. Acta Paediatr. 1994, 83, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, A.S.; Lenahan, J.L.; Izadnegahdar, R.; Ansermino, J.M. A Systematic Review of Tools to Measure Respiratory Rate in Order to Identify Childhood Pneumonia. Am. J. Respir. Crit. Care Med. 2018, 197, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chen, K.; Dai, Y.; Zhang, S. Utility of transthoracic impedance and novel algorithm for sleep apnea screening in pacemaker patient. In Sleep and Breathing; Springer: Berlin, Germany, 2018; pp. 1–6. [Google Scholar]
- Laine, J.; Mougenot, D. A high-sensitivity MEMS-based accelerometer. Lead. Edge 2014, 33, 1234–1242. [Google Scholar] [CrossRef]
- Bates, A.; Ling, M.J.; Mann, J.; Arvind, D. Respiratory rate and flow waveform estimation from tri-axial accelerometer data. In Proceedings of the 2010 International Conference on Body Sensor Networks, Singapore, 7–9 June 2010; pp. 144–150. [Google Scholar]
- Reinvuo, T.; Hannula, M.; Sorvoja, H.; Alasaarela, E.; Myllyla, R. Measurement of respiratory rate with high-resolution accelerometer and EMFit pressure sensor. In Proceedings of the 2006 IEEE Sensors Applications Symposium, Houston, TX, USA, 7–9 February 2006; pp. 192–195. [Google Scholar]
- Hung, P.; Bonnet, S.; Guillemaud, R.; Castelli, E.; Yen, P.T.N. Estimation of respiratory waveform using an accelerometer. In Proceedings of the 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro (ISBI 2008), Paris, France, 14–17 May 2008; pp. 1493–1496. [Google Scholar]
- Jin, A.; Yin, B.; Morren, G.; Duric, H.; Aarts, R.M. Performance evaluation of a tri-axial accelerometry-based respiration monitoring for ambient assisted living. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC 2009), Minneapolis, MN, USA, 3–6 September 2009; pp. 5677–5680. [Google Scholar]
- Fekr, A.R.; Janidarmian, M.; Radecka, K.; Zilic, Z. A medical cloud-based platform for respiration rate measurement and hierarchical classification of breath disorders. Sensors 2014, 14, 11204–11224. [Google Scholar] [CrossRef]
- Liu, G.Z.; Guo, Y.W.; Zhu, Q.S.; Huang, B.Y.; Wang, L. Estimation of respiration rate from three-dimensional acceleration data based on body sensor network. Telemed. e-Health 2011, 17, 705–711. [Google Scholar] [CrossRef]
- Vertens, J.; Fischer, F.; Heyde, C.; Hoeflinger, F.; Zhang, R.; Reindl, L.; Gollhofer, A. Measuring Respiration and Heart Rate using Two Acceleration Sensors on a Fully Embedded Platform. In Proceedings of the 3rd International Congress on Sport Sciences Research and Technology Support, Lisbon, Portugal, 15–17 November 2015; pp. 15–23. [Google Scholar]
- Passaro, V.; Cuccovillo, A.; Vaiani, L.; De Carlo, M.; Campanella, C.E. Gyroscope technology and applications: A review in the industrial perspective. Sensors 2017, 17, 2284. [Google Scholar] [CrossRef]
- Yoon, J.W.; Noh, Y.S.; Kwon, Y.S.; Kim, W.K.; Yoon, H.R. Improvement of dynamic respiration monitoring through sensor fusion of accelerometer and gyro-sensor. J. Electr. Eng. Technol. 2014, 9, 334–343. [Google Scholar] [CrossRef]
- Lee, H.J.; Jung, S. Gyro sensor drift compensation by Kalman filter to control a mobile inverted pendulum robot system. In Proceedings of the IEEE International Conference on Industrial Technology (ICIT 2009), Gippsland, VIC, Australia, 10–13 February 2009; pp. 1–6. [Google Scholar]
- Wang, S.; Liu, M.; Pang, B.; Li, P.; Yao, Z.; Zhang, X.; Chen, H. A new physiological signal acquisition patch designed with advanced respiration monitoring algorithm based on 3-axis accelerator and gyroscope. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 441–444. [Google Scholar]
- Pavlov, I. Selection of normalized metrological characteristics of rate gyros. Meas. Tech. 1993, 36, 680–681. [Google Scholar] [CrossRef]
- Yazdi, N.; Ayazi, F.; Najafi, K. Micromachined inertial sensors. Proc. IEEE 1998, 86, 1640–1659. [Google Scholar] [CrossRef]
- Shen, C.L.; Huang, T.H.; Hsu, P.C.; Ko, Y.C.; Chen, F.L.; Wang, W.C.; Kao, T.; Chan, C.T. Respiratory Rate Estimation by Using ECG, Impedance, and Motion Sensing in Smart Clothing. J. Med. Biol. Eng. 2017, 37, 826–842. [Google Scholar] [CrossRef] [PubMed]
- Milici, S.; Lázaro, A.; Villarino, R.; Girbau, D.; Magnarosa, M. Wireless Wearable Magnetometer-Based Sensor for Sleep Quality Monitoring. IEEE Sens. J. 2018, 18, 2145–2152. [Google Scholar] [CrossRef]
- Oh, Y.; Jung, Y.J.; Choi, S.; Kim, D. Design and Evaluation of a MEMS Magnetic Field Sensor-Based Respiratory Monitoring and Training System for Radiotherapy. Sensors 2018, 18, 2742. [Google Scholar] [CrossRef]
- McCool, F.D.; Wang, J.; Ebi, K.L. Tidal volume and respiratory timing derived from a portable ventilation monitor. Chest 2002, 122, 684–691. [Google Scholar] [CrossRef]
- Derchak, P.A.; Czapla, L.; Rogan, C.A. Magnetometer Based Physiological Monitoring Garment. U.S. Patent 9,801,583, 31 October 2017. [Google Scholar]
- Cesareo, A.; Previtali, Y.; Biffi, E.; Aliverti, A. Assessment of Breathing Parameters Using an Inertial Measurement Unit (IMU)-Based System. Sensors 2018, 19, 88. [Google Scholar] [CrossRef]
- Cesareo, A.; Gandolfi, S.; Pini, I.; Biffi, E.; Reni, G.; Aliverti, A. A novel, low cost, wearable contact-based device for breathing frequency monitoring. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Seogwipo, Korea, 11–15 July 2017; pp. 2402–2405. [Google Scholar]
- Charlton, P.H.; Birrenkott, D.A.; Bonnici, T.; Pimentel, M.A.; Johnson, A.E.; Alastruey, J.; Tarassenko, L.; Watkinson, P.J.; Beale, R.; Clifton, D.A. Breathing rate estimation from the electrocardiogram and photoplethysmogram: A review. IEEE Rev. Biomed. Eng. 2018, 11, 2–20. [Google Scholar] [CrossRef]
- Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable photoplethysmographic sensors—Past and present. Electronics 2014, 3, 282–302. [Google Scholar] [CrossRef]
- Goldman, M.J. Principles of Clinical Electrocardiography; Lange Medical Publications: New York, NY, USA, 1986. [Google Scholar]
- Khunti, K. Accurate interpretation of the 12-lead ECG electrode placement: A systematic review. Health Educ. J. 2014, 73, 610–623. [Google Scholar] [CrossRef]
- Jevon, P. Procedure for recording a standard 12-lead electrocardiogram. Br. J. Nurs. 2010, 19, 649–651. [Google Scholar] [CrossRef] [PubMed]
- Bailón, R.; Sörnmo, L.; Laguna, P. ECG derived respiratory frequency estimation - Chapter 8. In Advanced Methods and Tools for ECG Data Analysis; Artech House: London, UK, 2006; Volume 1. [Google Scholar]
- Berntson, G.G.; Cacioppo, J.T.; Quigley, K.S. Respiratory sinus arrhythmia: Autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology 1993, 30, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Tiinanen, S.; Noponen, K.; Tulppo, M.; Kiviniemi, A.; Seppänen, T. ECG-derived respiration methods: Adapted ICA and PCA. Med. Eng. Phys. 2015, 37, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Boehm, A.; Neu, W.; Venema, B.; Marx, N.; Leonhardt, S.; Teichmann, D. A wearable 12-lead ECG T-shirt with textile electrodes for unobtrusive long-term monitoring—Evaluation of an ongoing clinical trial. In EMBEC & NBC 2017; Springer: Berlin, Germany, 2017; pp. 703–706. [Google Scholar]
- Chi, Y.M.; Jung, T.P.; Cauwenberghs, G. Dry-contact and noncontact biopotential electrodes: Methodological review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.N.; Das, R.; Pal, S. Automated Real-Time Processing of Single Lead Electrocardiogram for Simultaneous Heart Rate and Respiratory Rate Monitoring. J. Med. Devices 2017, 11, 024502. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.; Schumann, A.; Müller, J.; Bär, K.J.; Rose, G. ECG derived respiration: Comparison of time-domain approaches and application to altered breathing patterns of patients with schizophrenia. Physiol. Meas. 2017, 38, 601. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, I.; Noponen, K.; Hautala, A.; Ammann, R.; Seppänen, T. Spectral fusion-based breathing frequency estimation; experiment on activities of daily living. Biomed. Eng. Online 2018, 17, 99. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, H.; Xu, Z.; Xiao, M.; Song, J. A principal component analysis based data fusion method for ECG-derived respiration from single-lead ECG. Australas. Phys. Eng. Sci. Med. 2018, 41, 59–67. [Google Scholar] [CrossRef]
- Allen, J. Photoplethysmography and its application in clinical physiological measurement. Physiol. Meas. 2007, 28. [Google Scholar] [CrossRef]
- Meredith, D.; Clifton, D.; Charlton, P.; Brooks, J.; Pugh, C.; Tarassenko, L. Photoplethysmographic derivation of respiratory rate: A review of relevant physiology. J. Med. Eng. Technol. 2012, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, M.; Faib, I.; Friedman, H. Respiration-induced changes in tissue blood volume distal to occluded artery, measured by photoplethysmography. J. Biomed. Opt. 2006, 11, 040506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lázaro, J.; Gil, E.; Bailón, R.; Mincholé, A.; Laguna, P. Deriving respiration from photoplethysmographic pulse width. Med. Biol. Eng. Comput. 2013, 51, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Moraes, J.; Rocha, M.; Vasconcelos, G.; Vasconcelos Filho, J.; de Albuquerque, V. Advances in photopletysmography signal analysis for biomedical applications. Sensors 2018, 18, 1894. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Chung, H.; Lee, J. Motion Artifact Cancellation in Wearable Photoplethysmography Using Gyroscope. IEEE Sens. J. 2019, 19, 1166–1175. [Google Scholar] [CrossRef]
- Touw, H.R.; Verheul, M.H.; Tuinman, P.R.; Smit, J.; Thöne, D.; Schober, P.; Boer, C. Photoplethysmography respiratory rate monitoring in patients receiving procedural sedation and analgesia for upper gastrointestinal endoscopy. J. Clin. Monit. Comput. 2017, 31, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Bergese, S.D.; Mestek, M.L.; Kelley, S.D.; McIntyre, R., Jr.; Uribe, A.A.; Sethi, R.; Watson, J.N.; Addison, P.S. Multicenter study validating accuracy of a continuous respiratory rate measurement derived from pulse oximetry: A comparison with capnography. Anesth. Analg. 2017, 124, 1153. [Google Scholar] [CrossRef]
- Shah, S.A.; Fleming, S.; Thompson, M.; Tarassenko, L. Respiratory rate estimation during triage of children in hospitals. J. Med. Eng. Technol. 2015, 39, 514–524. [Google Scholar] [CrossRef]
- Sharma, H.; Sharma, K. ECG-derived respiration using Hermite expansion. Biomed. Signal Process. Control 2018, 39, 312–326. [Google Scholar] [CrossRef]










| Sensors | Metrological Properties | Sensor Characteristics | Applications |
|---|---|---|---|
| Differential flowmeters | ✓ Sensitivity * ✓ Step response time ✓/× Output linearity ** ✓ Accuracy | ∼ Sensor size ∼ Cost ✓ Real-time monitoring ∼ Measurement intrusiveness ✓ Sensitivity to body motion artifacts ✓ Influence of environmental factors × Presence of wire | Apnea SB QB FB |
| Turbine flowmeters | ✓ Sensitivity ✓ Step response time ✓ Output linearity ✓ Accuracy | ✓/∼ Sensor size * ∼ Cost ✓ Real-time monitoring ∼ Measurement intrusiveness ∼ Sensitivity to body motion artifacts ✓ Influence of environmental factors ∼ Presence of wire | Apnea SB QB FB |
| Hot wire anemometers | ✓ Sensitivity ✓ Step response time × Output linearity ✓ Accuracy | ∼ Sensor size ∼ Cost ✓ Real-time monitoring ∼ Measurement intrusiveness × Sensitivity to body motion artifacts ✓ Influence of environmental factors ∼ Presence of wire | Apnea SB QB FB |
| Fiber-optic sensors | ✓ Sensitivity ✓ Step response time ✓ Output linearity ✓ Accuracy | ✓ Sensor size × Cost ✓ Real-time monitoring ∼ Measurement intrusiveness × Sensitivity to body motion artifacts ∼ Influence of environmental factors × Presence of wire | Apnea SB QB FB |
| Sensors | Metrological Properties | Sensor Characteristics | Applications |
|---|---|---|---|
| Microphones | ✓ Sensitivity ✓ Step response time ✓/× Output linearity * ✓ Accuracy | ✓ Sensor size ✓ Cost ∼ Real-time monitoring ✓ Measurement intrusiveness ✓ Sensitivity to body motion artifacts × Influence of environmental factors ∼ Presence of wire ** | Apnea SB QB FB |
| Sensors | Metrological Properties | Sensor Characteristics | Applications |
|---|---|---|---|
| Thermistors | ✓ Sensitivity ∼ Step response time ✓ Output linearity ✓ Accuracy | ∼ Sensor size ✓ Cost ∼ Real-time monitoring * ∼ Measurement intrusiveness ✓ Sensitivity to body motion artifacts × Influence of environmental factors × Presence of wire | Apnea SB |
| Thermocouples | ✓ Sensitivity ✓ Step response time ✓ Output linearity ✓ Accuracy | ✓ Sensor size ∼ Cost ✓ Real-time monitoring ∼ Measurement intrusiveness ✓ Sensitivity to body motion artifacts × Influence of environmental factors × Presence of wire | Apnea SB QB FB |
| Pyroelectric sensors | ✓ Sensitivity ✓ Step response time ✓ Output linearity ✓ Accuracy | ✓ Sensor size ∼ Cost ✓ Real-time monitoring ∼ Measurement intrusiveness ✓ Sensitivity to body motion artifacts × Influence of environmental factors × Presence of wire | Apnea SB QB FB |
| Fiber-optic sensors | ✓ Sensitivity ✓ Step response time ✓ Output linearity ✓ Accuracy | ✓ Sensor size × Cost ** ✓ Real-time monitoring ∼ Measurement intrusiveness ✓ Sensitivity to body motion artifacts × Influence of environmental factors × Presence of wire | Apnea SB QB FB |
| Sensors | Metrological Properties | Sensor Characteristics | Applications |
|---|---|---|---|
| Capacitive sensors | ✓ Sensitivity × Step response time * ✓ Output linearity ✓ Accuracy | ∼ Sensor size ✓ Cost ✓ Real-time monitoring ∼ Measurement intrusiveness ✓ Sensitivity to body motion artifacts × Influence of environmental factors × Presence of wire | Apnea SB QB ** |
| Resistive sensors | ✓ Sensitivity ∼/× Step response time *** ✓ Output linearity ✓ Accuracy | ∼ Sensor size ✓ Cost ✓ Real-time monitoring ∼ Measurement intrusiveness ✓ Sensitivity to body motion artifacts × Influence of environmental factors × Presence of wire | Apnea SB QB ** FB ** |
| Nanocrystals and nanoparticles sensors | ✓ Sensitivity ✓/∼ Step response time **** ✓ Output linearity ✓ Accuracy | ∼ Sensor size ✓ Cost ✓ Real-time monitoring ∼ Measurement intrusiveness ✓ Sensitivity to body motion artifacts × Influence of environmental factors × Presence of wire | Apnea SB QB FB ** |
| Fiber-optic sensors | ✓ Sensitivity ✓/∼ Step response time ✓ Output linearity ✓ Accuracy | ✓ Sensor size × Cost ✓ Real-time monitoring ∼ Measurement intrusiveness ∼ Sensitivity to body motion artifacts ∼ Influence of environmental factors × Presence of wire | Apnea SB QB FB |
| Sensors | Metrological Properties | Sensor Characteristics | Applications |
|---|---|---|---|
| Infrared sensors | ✓ Sensitivity ✓ Step response time ✓ Output linearity ✓ Accuracy | ∼ Sensor size ∼ Cost ✓ Real-time monitoring ∼ Measurement intrusiveness ✓ Sensitivity to body motion artifacts × Influence of environmental factors × Presence of wire | Apnea SB QB FB |
| Fiber-optic sensors | ✓ Sensitivity ✓ Step response time ✓ Output linearity ✓ Accuracy | ✓ Sensor size ∼/× Cost * ✓ Real-time monitoring ∼ Measurement intrusiveness ✓ Sensitivity to body motion artifacts × Influence of environmental factors × Presence of wire | Apnea SB QB FB |
| Sensors | Metrological Properties | Sensor Characteristics | Applications |
|---|---|---|---|
| Resistive sensors | ✓ Sensitivity ✓ Step response time ∼ Output linearity * ✓ Accuracy | ✓ Sensor size ✓ Cost ✓ Real-time monitoring ✓/∼ Measurement intrusiveness × Sensitivity to body motion artifacts ∼ Influence of environmental factors ✓ Presence of wire | Apnea SB QB FB |
| Capacitive sensors | ✓ Sensitivity ✓ Step response time ∼ Output linearity ✓ Accuracy | ✓ Sensor size ✓ Cost ✓ Real-time monitoring ✓/∼ Measurement intrusiveness × Sensitivity to body motion artifacts ✓ Influence of environmental factors ✓ Presence of wire | Apnea SB QB FB |
| Inductive sensors | ✓ Sensitivity ✓ Step response time ∼ Output linearity ✓ Accuracy | ∼ Sensor size (around the chest) ✓ Cost ✓ Real-time monitoring ✓/∼ Measurement intrusiveness ∼ Sensitivity to body motion artifacts ✓ Influence of environmental factors ∼ Presence of wire | Apnea SB QB FB |
| Fiber-optic sensors | ✓ Sensitivity ✓ Step response time ∼ Output linearity ✓ Accuracy | ✓ Sensor size ∼ Cost ** ✓ Real-time monitoring ✓/∼ Measurement intrusiveness × Sensitivity to body motion artifacts ✓ Influence of environmental factors ∼ Presence of wire *** | Apnea SB QB FB |
| Sensors | Metrological Properties | Sensor Characteristics | Applications |
|---|---|---|---|
| Impedance sensors | ✓ Sensitivity ✓ Step response time ✓ Output linearity ✓ Accuracy | ✓ Sensor size ✓ Cost ✓ Real-time monitoring ∼ Measurement intrusiveness × Sensitivity to body motion artifacts ✓ Influence of environmental factors ∼ Presence of wire * | Apnea SB QB FB |
| Sensors | Metrological properties | Sensor characteristics | Applications |
|---|---|---|---|
| Accelerometers | ✓ Sensitivity ✓ Step response time ✓ Output linearity ✓ Accuracy | ✓ Sensor size ✓ Cost ✓ Real-time monitoring ✓ Measurement intrusiveness × Sensitivity to body motion artifacts ✓ Influence of environmental factors ✓ Presence of wire | Apnea SB QB FB |
| Gyroscopes | ✓ Sensitivity ✓ Step response time ✓ Output linearity ✓ Accuracy | ✓ Sensor size ✓ Cost ✓ Real-time monitoring ✓ Measurement intrusiveness × Sensitivity to body motion artifacts ✓ Influence of environmental factors ✓ Presence of wire | Apnea SB QB FB |
| Magnetometers | ✓ Sensitivity ✓ Step response time ✓ Output linearity ✓ Accuracy | ✓ Sensor size ✓ Cost ✓ Real-time monitoring ✓ Measurement intrusiveness × Sensitivity to body motion artifacts ∼ Influence of environmental factors ✓ Presence of wire | Apnea SB QB FB |
| Sensors | Metrological Properties | Sensor Characteristics | Applications |
|---|---|---|---|
| ECG sensors | ✓ Sensitivity ✓ Step response time ✓ Output linearity ∼ Accuracy * | ✓ Sensor size ∼ Cost * ✓ Real-time monitoring ∼ Measurement intrusiveness ∼ Sensitivity to body motion artifacts ∼ Influence of environmental factors ∼ Presence of wire ** | Apnea SB QB FB |
| PPG sensors | ✓ Sensitivity ✓ Step response time ✓ Output linearity ∼ Accuracy | ✓ Sensor size ✓ Cost ✓ Real-time monitoring ✓ Measurement intrusiveness × Sensitivity to body motion artifacts *** ✓ Influence of environmental factors ✓ Presence of wire | Apnea SB QB FB |
| CLINICAL | SETTINGS | OCCUPATIONAL | SETTINGS | SPORT AND | EXERCISE | |||
|---|---|---|---|---|---|---|---|---|
| CONTACT-BASED TECHNIQUE | A | B | A | B | A | B | Main Advantages | Main Disadvantages |
| Respiratory airflow | ✓ | ∼ | ✓ | ∼ | ✓ | ∼ | Accuracy | Intrusiveness |
| Respiratory sounds | ∼ | ∼ | ∼ | × | ∼ | × | Unobtrusiveness | Environmental influence |
| Air temperature | ✓ | ∼ | ∼ | × | ∼ | × | Sensitivity | Intrusiveness |
| Air humidity | ∼ | ∼ | ∼ | × | ∼ | × | Low sensitivity to motion artifacts | Intrusiveness |
| Air components | ✓ | ∼ | ✓ | × | ∼ | × | Accuracy | Intrusiveness |
| Strain measurements | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | Unobtrusiveness | Motion artifacts |
| Impedance measurements | ✓ | ∼ | ✓ | × | ∼ | × | Unobtrusiveness | Motion artifacts |
| Movement measurements | ∼ | ∼ | ∼ | ∼ | ∼ | × | Unobtrusiveness | Motion artifacts |
| Biopotential measurements (i.e., ECG) | ✓ | ∼ | ∼ | × | ∼ | × | Unobtrusiveness | Motion artifacts |
| Light intensity measurements (i.e., PPG) | ✓ | ∼ | ∼ | × | ∼ | × | Unobtrusiveness | Motion artifacts |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massaroni, C.; Nicolò, A.; Lo Presti, D.; Sacchetti, M.; Silvestri, S.; Schena, E. Contact-Based Methods for Measuring Respiratory Rate. Sensors 2019, 19, 908. https://0-doi-org.brum.beds.ac.uk/10.3390/s19040908
Massaroni C, Nicolò A, Lo Presti D, Sacchetti M, Silvestri S, Schena E. Contact-Based Methods for Measuring Respiratory Rate. Sensors. 2019; 19(4):908. https://0-doi-org.brum.beds.ac.uk/10.3390/s19040908
Chicago/Turabian StyleMassaroni, Carlo, Andrea Nicolò, Daniela Lo Presti, Massimo Sacchetti, Sergio Silvestri, and Emiliano Schena. 2019. "Contact-Based Methods for Measuring Respiratory Rate" Sensors 19, no. 4: 908. https://0-doi-org.brum.beds.ac.uk/10.3390/s19040908