Enabling Medicine Reuse Using a Digital Time Temperature Humidity Sensor in an Internet of Pharmaceutical Things Concept
Abstract
:1. Introduction
2. Methods
2.1. Design Criterion
- (i)
- Temperature
- (ii)
- Relative humidity
- (iii)
- Light intensity
- (vi)
- Agitation
- (v)
- Contamination
2.2. Design Requirement Recommendations
- (1)
- Environmental Parameter Sensing: Related to the monitoring and recording of environmental parameters regarding package surroundings, the sensing range, accuracy and sampling frequency are major concerns.
- (2)
- Expiry Date Monitoring and Management: Related to the functions of setting, resetting and alerting regarding a dynamic expiry date system.
- (3)
- Safety Protection: Related to the algorithms protecting medicines from tampering and counterfeiting.
- (4)
- Internet Connectivity: Related to the ways connecting to the Internet wirelessly.
- (5)
- Cloud Computing: Related to the web services empowering various data analytics for sharing specific information to stakeholders.
- (6)
- Size and Power Constraints: Related to the limitations concerning product sizes and power consumptions of technology embedded smart sensors.
- (7)
- Usability: Related to the user interfaces aiming for easy to use and easy to learn ways of validating unused medicines.
- (8)
- Cost Effectiveness: Related to the effective cost of implementing the whole IoPT architecture and its components.
- (9)
- Incentive Facilitation: Related to the administration of stakeholders’ incentives for motivating positive behaviours for reusing medicines.
- (10)
- Social Norms Support: Related to the setup of digital social norms shaping stakeholders’ behaviours normative beliefs.
- (11)
- Security and Privacy Protection: Related to the protection against unauthorised access to all data of the prescribed medicines through provision of end to end protection.
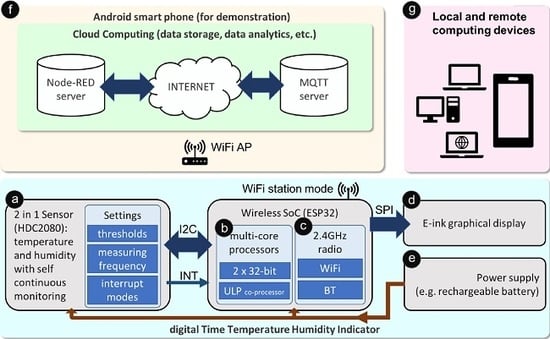
3. Results
- ⓐ
- A low power environmental sensor that performs continuous sensing and triggering functions without an external controller to save energy.
- ⓑ
- A main MCU which infrequently reads sensor data and uploads results to the Internet for cloud computing, it also updates the on-packaging display for zero distance local connectivity.
- ⓒ
- A wireless module which provides Internet connectivity through a wireless local area network.
- ⓓ
- An ultra-low power graphical display module that indicates the current sensing results as well as the quality status of the packaged medicines.
- ⓔ
- An energy supply based on a lithium battery that maintains the operations of the smart sensor.
- ⓕ
- A cloud computing platform that stores sensing data collected from the smart sensor and allows sharing of information to all stakeholders through various web services.
- ⓖ
- Computing devices (e.g., desktop computers, laptop computers, smartphones, etc.) that connect to cloud servers and retrieve bespoken information from anywhere with Internet connectivity.
3.1. Firmware Implementation
4. Discussions
4.1. Environmental Parameter Sensing
4.2. Expiry Date Monitoring and Management
4.3. Safety Protection
4.4. Internet Connectivity
4.5. Cloud Computing
4.6. Size and Power Consumption
4.7. Usability
4.8. Cost Effectiveness
4.9. Incentive Facilitation
4.10. Social Norms Support
4.11. Security and Privacy Protection
4.12. Alternative Solutions
4.13. Future Development Suggestions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Trueman, P.; Taylor, D.; Lowson, K.; Bligh, A.; Meszaros, A.; Wright, D.; Glanville, J.; Newbould, J.; Bury, M.; Barber, N.; et al. Evaluation of the Scale, Causes and Costs of Waste Medicines, Technical Report, DH Funded National Project. 2010. Available online: https://discovery.ucl.ac.uk/id/eprint/1350234/1/Evaluation_of_NHS_Medicines_Waste__web_publication_version.pdf (accessed on 15 April 2020).
- Singleton, J.A.; Nissen, L.M.; Barter, N.; McIntosh, M. The global public health issue of pharmaceutical waste: What role for pharmacists? J. Glob. Responsib. 2014, 5, 126–137. [Google Scholar] [CrossRef] [Green Version]
- Hazell, B.; Robson, R. Pharmaceutical Waste Reduction in the NHS. Available online: https://www.england.nhs.uk/wp-content/uploads/2015/06/pharmaceutical-waste-reduction.pdf (accessed on 15 April 2020).
- Connelly, D. Should pharmacists be allowed to reuse medicines. Pharm. J. 2019. [Google Scholar] [CrossRef]
- McRae, D.; Allman, M.; James, D. The redistribution of medicines: Could it become a reality? Int. J. Pharm. Pract. 2016, 24, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Bekker, C.L.; Gardarsdottir, H.; Egberts, T.C.G.; Bouvy, M.L.; van den Bemt, B.J.F. Redispensing of medicines unused by patients: A qualitative study among stakeholders. Int. J. Clin. Pharm. 2017, 39, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Bekker, C.; van den Bemt, B.; Egberts, T.C.G.; Bouvy, M.; Gardarsdottir, H. Willingness of patients to use unused medication returned to the pharmacy by another patient: A cross-sectional survey. BMJ Open 2019, 9, e024767. [Google Scholar] [CrossRef]
- Alhamad, H.; Patel, N.; Donyai, P. How do people conceptualise the reuse of medicines? An interview study. Int. J. Pharm. Pract. 2018, 26, 232–241. [Google Scholar] [CrossRef]
- Hui, T.K.L.; Mohammed, B.; Donyai, P.; McCrindle, R.; Sherratt, R.S. Enhancing pharmaceutical packaging through a technology ecosystem to facilitate the reuse of medicines and reduce medicinal waste. Pharmacy 2020, 8, 58. [Google Scholar] [CrossRef] [Green Version]
- Kuswandi, B.; Wicaksono, Y.; Jayus Abdullah, A.; Lee, Y.H.; Ahmad, M. Smart packaging: Sensors for monitoring of food quality and safety. Sens. Instrum. Food Qual. Saf. 2011, 5, 137–146. [Google Scholar] [CrossRef]
- Sehgal, S.; Jaithliya, T.; Khan, M.; Devi, A.N.; Banoo, J.; Tiwari, A. Recent trends and future of pharmaceutical packaging technology: An overview. Eur. J. Biomed. Pharm. Sci. 2018, 5, 957–966. [Google Scholar]
- Christensson, P. SMART (Self-Monitoring Analysis And Reporting Technology) Definition. Available online: https://techterms.com/definition/smart (accessed on 18 May 2020).
- Lorenzini, G.C.; Mostaghel, R.; Hellstrom, D. Drivers of pharmaceutical packaging innovation: A customer-supplier relationship case study. J. Bus. Res. 2018, 88, 363–370. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Yang, M.; Zhang, Y.; Xiang, K.; Tang, R. Review of time temperature indicators as quality monitors in food packaging. Packag. Technol. Sci. 2015, 28, 839–867. [Google Scholar] [CrossRef]
- Mijanur Rahman, A.T.M.; Kim, D.H.; Jang, H.D.; Yang, J.H.; Lee, S.J. Preliminary study on biosensor-type time-temperature integrator for intelligent food packaging. Sensors 2018, 18, 1949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Shan, X.; Wei, J. Hybrid Flexible Smart Temperature Tag with NFC Technology for Smart Packaging. In Proceedings of the IEEE 19th Electronics Packaging Technology Conference, Singapore, 6–9 December 2017. [Google Scholar]
- Madhusudan, P.; Chellukuri, N.; Shivakumar, N. Smart packaging of food for the 21st century—A review with futuristic trends, their feasibility and economics. Mater. Today Proc. 2018, 5, 21018–21022. [Google Scholar] [CrossRef]
- Kuswandi, B.; Moradi, M. Sensor trends in beverages packaging. Trends Beverage Packag. 2019, 16, 279–302. [Google Scholar] [CrossRef]
- Johnston, J.J.; Wong, J.P.; Feldman, S.E.; Ilnicki, L.P. Purge and trap/gas chromatography/mass spectrometry method for determining smoke contamination of foods and packaging materials. J. Agric. Food Chem. 1994, 42, 1954–1958. [Google Scholar] [CrossRef]
- Colberg, L.; Schmidt-Petersen, L.; Hansen, M.K.; Larsen, B.S.; Otnes, S. Incorrect storage of medicines and potential for cost savings. Eur. J. Hosp. Pharm. 2017, 24, 167–169. [Google Scholar] [CrossRef]
- Kumar, A.K.; Gupta, N.V.; Lalasa, P.; Sandhil, S. A review on packaging materials with anti-counterfeit, tamper-evident features for pharmaceuticals. Int. J. Drug Dev. Res. 2013, 5, 26–34. [Google Scholar]
- Yamin, P.; Fei, M.; Lahlou, S.; Levy, S. Using social norms to change behavior and increase sustainability in the real world: A systematic review of the literature. Sustainability 2019, 11, 5847. [Google Scholar] [CrossRef] [Green Version]
- Maity, M.; Bagchi, K.; Shah, A.; Misra, A. Explaining normative behavior in information technology use. Inf. Technol. People 2019, 32, 94–117. [Google Scholar] [CrossRef]
- Piyare, R.; Murphy, A.L.; Kiraly, C.; Tosato, P.; Brunelli, D. Ultra low power wake-up radios: A hardware and networking survey. IEEE Commun. Surv. Tutor. 2017, 19, 2117–2157. [Google Scholar] [CrossRef]
- Jang, T.; Choi, M.; Shi, Y.; Lee, I.; Sylvester, D.; Blaauw, D. Millimeter-Scale Computing Platform for Next Generation of Internet of Things. In Proceedings of the RFID 2016: International IEEE Conference on RFID, Orlando, FL, USA, 3–5 May 2016; pp. 1–4. [Google Scholar] [CrossRef]
- Hui, T.K.L.; Sherratt, R.S. Towards disappearing user interfaces for ubiquitous computing: Human enhancement from sixth sense to super senses. J. Ambient. Intell. Humaniz. Comput. 2017, 8, 449–465. [Google Scholar] [CrossRef] [Green Version]
- Koomey, J.; Naffziger, S. Moore’s Law might be slowing down, but not energy efficiency. IEEE spectrum 2015, 52, 35. [Google Scholar]
- Texas Instruments. Low-Power Humidity and Temperature Digital Sensor. Available online: http://www.ti.com/lit/ds/symlink/hdc2080.pdf (accessed on 15 April 2020).
- Espressif Systems. ESP32 Series Datasheet. Available online: https://www.espressif.com/sites/default/files/documentation/esp32_datasheet_en.pdf. (accessed on 15 April 2020).
- Waveshare Electronics. Specification EPD Screen Size: 2.13", Color: Black and White, Display Resolution: 250*122. 2017. Available online: https://www.waveshare.com/w/upload/e/e6/2.13inch_e-Paper_Datasheet.pdf. (accessed on 15 April 2020).
- Taylor, J. Recommendations on the control and monitoring of storage and transportation temperatures of medicinal products. Pharm. J. 2001, 267, 128–131. [Google Scholar]
- Fu, M.; Perlman, M.; Lu, Q.; Varga, C. Pharmaceutical solid-state kinetic stability investigation by using moisture-modified Arrhenius equation and JMP statistical software. J. Pharm. Biomed. Anal. 2015, 107, 370–377. [Google Scholar] [CrossRef] [PubMed]
- NHS England. Novel Coronavirus (COVID-19) Standard Operating Procedure: Running a Medicines Re-Use Scheme in a Care Home or Hospice Setting. Report; 2020. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/881838/medicines-reuse-in-care-homes.pdf (accessed on 18 May 2020).
- Boyd, M. Council Approves Use of Patient-Returned and Date-Expired Medicines in the Event of Pandemic Flu. Available online: https://www.pharmaceutical-journal.com/news-and-analysis/council-approves-use-of-patient-returned-and-date-expired-medicines-in-the-event-of-pandemic-flu/10036098.article (accessed on 18 May 2020).
- Nørfeldt, L.; Bøtker, J.; Edinger, M.; Genina, N.; Rantanen, J. Cryptopharmaceuticals: Increasing the safety of medication by a blockchain of pharmaceutical products. J. Pharm. Sci. 2019, 108, 2838–2841. [Google Scholar] [CrossRef]
- Andreev, S.; Galinina, O.; Pyattaev, A.; Gerasimenko, M.; Tirronen, T.; Torsner, J.; Sachs, J.; Dohler, M.; Koucheryavy, Y. Understanding the IoT connectivity landscape: A contemporary M2M radio technology roadmap. IEEE Commun. Mag. 2015, 53, 32–40. [Google Scholar] [CrossRef] [Green Version]
- Norman, D. The Design of Everyday Things: Revised and Expanded Edition. Basic Books. 2013. Available online: https://www.basicbooks.com/titles/don-norman/the-design-of-everyday-things/9780465050659/ (accessed on 18 May 2020).
- Viegas, C.V.; Bond, A.; Vaz, C.R.; Bertolo, R.J. Reverse flows within the pharmaceutical supply chain: A classificatory review from the perspective of end-of-use and end-of-life medicines. J. Clean. Prod. 2019, 238, 117719. [Google Scholar] [CrossRef]
- Bekker, C.L.; Gardarsdottir, H.; Egberts, A.C.G.; Molenaar, H.A.; Bouvy, M.L.; van den Bemt, B.J.F.; Hövels, A.M. What does it cost to redispense unused medications in the pharmacy? A micro-costing study. Bmc Health Serv. Res. 2019, 19, 243. [Google Scholar] [CrossRef]
- European Commission. Circular Economy Action Plan: For a Cleaner and More Competitive Europe. Report. 2020. Available online: https://ec.europa.eu/environment/circular-economy/pdf/new_circular_economy_action_plan.pdf (accessed on 18 May 2020).
- Black, G. Reuse of medicine: It’s not about the money!: From my Little Black Book of pharmacy practice: Practice matters. Pharm. J. 2011, 78, 51–53. [Google Scholar]
- Spottswood, E.L.; Hancock, J.T. Should I share that? Prompting social norms that influence privacy behaviors on a social networking site. J. Comput. Mediat. Commun. 2017, 22, 55–70. [Google Scholar] [CrossRef]
- Karsai, G.; Balasubramanian, D.; Dubey, A.; Otte, W.R. Distributed and Managed: Research Challenges and Opportunities of the Next Generation Cyber-Physical Systems. In Proceedings of the 17th IEEE International Symposium on Object/Component/Service-Oriented Real-Time Distributed Computing (ISORC), Reno, NV, USA, 10–12 June 2014; pp. 1–8. [Google Scholar] [CrossRef] [Green Version]
- Geismann, J.; Gerking, C.; Bodden, E. Towards Ensuring Security by Design in Cyber-Physical Systems Engineering Processes. In Proceedings of the International Conference on Software and System Process (ICSSP), Gothenburg, Sweden, 26–27 May 2018; pp. 123–127. [Google Scholar] [CrossRef]
- Rashid, M.; Imran, M.; Jafri, A.R. Comparative Analysis of Fexible Cryptographic Implementations. In Proceedings of the 11th International Symposium on Reconfigurable Communication-Centric Systems-on-Chip (ReCoSoC), Tallinn, Estonia, 27–29 June 2016. [Google Scholar] [CrossRef]
- Wang, J.; Wu, W.; Liao, Z.; Sherratt, R.S.; Kim, G.; Alfarraj, O.; Alzubi, A.; Tolba, A. A probability preferred priori offloading mechanism in mobile edge computing. IEEE Access 2020, 8, 39758–39767. [Google Scholar] [CrossRef]
- Farahani, B.; Firouzi, F.; Chang, V.; Badaroglu, M.; Constant, N.; Mankodiya, K. Towards fog-driven IoT eHealth: Promises and challenges of IoT in medicine and healthcare. Future Gener. Comput. Syst. 2018, 78, 659–676. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.C.; Liu, Z.G.; Ndibanje, B.; Nkenyereye, L.; Riazul Islam, S. An IoT-based anonymous function for security and privacy in healthcare sensor networks. Sensors 2019, 19, 3146. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yang, Y.; Wang, T.; Sherratt, R.S.; Zhang, J. Big Data Service Architecture: A Survey. J. Internet Technol. 2020, 21, 393–405. [Google Scholar] [CrossRef]
- Abouelmehdi, K.; Beni-Hessane, A.; Khaloufi, H. Big healthcare data: Preserving security and privacy. J. Big Data 2018, 5, 1. [Google Scholar] [CrossRef]
- Dwivedi, A.D.; Srivastava, G.; Dhar, S.; Singh, R. A decentralized privacy-preserving healthcare blockchain for IoT. Sensors 2019, 19, 326. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Xie, L.; Mantysalo, M.; Zhou, X.L.; Pang, Z.B.; Xu, L.D.; Kao-Walter, S.; Chen, Q.; Zheng, L.R. A health-IoT platform based on the integration of intelligent packaging, unobtrusive bio-sensor, and intelligent medicine box. IEEE Trans. Ind. Informatics 2014, 10, 2180–2191. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.; Tseng, C.H.; Wang, L.; Juang, F. Bidirectional Smart Pill Box Monitored through Internet and Receiving Reminding Message from Remote Relatives. In Proceedings of the IEEE International Conference on Consumer Electronics-Taiwan (ICCE-TW), Taipei, Taiwan, 12–14 June 2017; pp. 393–394. [Google Scholar] [CrossRef]
- Abdul Minaam, D.S.; Abd-Elfattah, M. Smart drugs: Improving healthcare using smart pill box for medicine reminder and monitoring system. Future Comput. Inform. J. 2018, 3, 443–456. [Google Scholar] [CrossRef]
- Donyai, P.; McCrindle, R.; Sherratt, R.S.; Hui, T.K.L. COVID-19 Pandemic Is Our Chance to Learn How to Reuse Old Medicines. Available online: https://theconversation.com/covid-19-pandemic-is-our-chance-to-learn-how-to-reuse-old-medicines-137671?utm_source=twitter&utm_medium=bylinetwitterbutton. (accessed on 14 May 2020).
- Bjerke, M.B.; Renger, R. Being smart about writing SMART objectives. Eval. Prog. Plan. 2017, 61, 125–127. [Google Scholar] [CrossRef]






| Qualitative Research | |
| Quality Requirements |
|
| Safety Requirements |
|
| Other Requirements |
|
| Proposed Development Requirements and Suggested Future Research | SMART Goal Setting Suggestions(S: Specific, M: Measurable, A: Attainable, R: Realistic, T: Timely) |
|---|---|
Environmental parameter sensing:
| S: extract validation guidelines from existing standard operating procedures from medical professionals, government and pharmaceutical companies. M: separate each sensor design into controllable phases. A: only existing technologies are chosen. R: using existing standards from pharmaceutical companies for every type of medicines as sensing target. T: divide whole project into several phases to produce timely outcomes. |
Expiry date monitoring and management:
| S: define a dynamic expiry date system with remote access through Internet connectivity, and the rules to change can be configurable at any time after the system is complete. M: a clearly defined design specification can be used as validation tool. A: using existing IoT technologies. R: using existing IoT technologies. T: divide whole project into several phases to produce timely outcomes. |
Safety protection:
| S: for tampering, need to define the types of tampering for study to limit the scope since there are too many tampering methods being used; for anti-counterfeit, try to expand the existing technology to include medicines as part of the system. M: separate goal into achievable objectives. A: based on application of existing technologies. R: based on application of existing technologies. T: divide whole project into several phases to produce timely outcomes. |
Internet connectivity:
| S: choose existing technology according to specific requirements (automatic roaming, low cost, low power, etc.). M: using existing IoT technologies. A: using existing IoT technologies. R: using existing IoT technologies. T: divide whole project into several phases to produce timely outcomes. |
Cloud computing:
| S: choose existing cloud computing technology according to specific requirements (extensible and reconfigurable cloud computing platform). M: using existing IoT technologies. A: using existing IoT technologies. R: using existing IoT technologies. T: divide whole project into several phases to produce timely outcomes. |
Size and power consumption:
| S: need to first define the maximum size and power budget before conducting any study, may provide several class of solutions to fit for different medicinal products if no single architecture can resolve all concerns. M: separate goal into achievable objectives. A: based on application of existing technologies. R: based on application of existing technologies. T: divide whole project into several phases to produce timely outcomes. |
Usability:
| S: user survey is critical in defining the minimum requirements for user interfaces that facilitate medicine reuse through shaping patients’ behaviours, it also affects the form factor of indications and the context to be delivered to stakeholders. M: separate goal into achievable objectives. A: based on application of existing technologies. R: based on application of existing technologies. T: divide whole project into several phases to produce timely outcomes. |
Cost effectiveness:
| S: a detail study is conducted initially to define the way for sharing the project cost, and a reasonable estimation of annual saving is proposed as the baseline to derive the individual budgets for each sub-system. This exercise is the first step in building the ReMINDS ecosystem based on the IoPT concept, thus, it is of the highest priority. the highest M: estimation based on empirical data from previous research and current quantitative study from the pharmaceutical sector. A: based on application of existing technologies. R: based on application of existing technologies. T: divide whole project into several phases to produce timely outcomes. |
Incentive facilitation:
| S: conduct cross-disciplinary qualitative research to define what incentive schemes are acceptable to the stakeholders and define the criterion for implementing the scheme on cloud computing. M: using existing IoT technologies. A: using existing IoT technologies. R: using existing IoT technologies. T: divide whole project into several phases to produce timely outcomes. |
Social norms support:
| S: conduct cross-disciplinary qualitative research to define what social norms settings are appropriate to motivate stakeholders’ behaviours and define the criterion for implementing the digital social norms network on cloud computing. M: using existing IoT technologies. A: using existing IoT technologies. R: using existing IoT technologies. T: divide whole project into several phases to produce timely outcomes. |
Security and privacy protection:
| S: try to expand the existing technology, especially big data for healthcare and blockchain technologies, to include medicines as part of the security and privacy protection system. M: separate goal into achievable objectives. A: based on application of existing technologies. R: based on application of existing technologies. T: divide whole project into several phases to produce timely outcomes. |
| Conflicts Between Design Requirements | Environmental Parameter Sensing | Expiry Date Monitoring and Management | Safety Protection | Internet Connectivity | Cloud Computing | Size and Power Consumption | Usability | Cost Effectiveness | Incentive Facilitation | Social Norms Support | Security and Privacy Protection |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Environmental parameter sensing | X | X | |||||||||
| Expiry date monitoring and management | X | X | |||||||||
| Safety protection | X | X | |||||||||
| Internet connectivity | X | X | X | ||||||||
| Cloud computing | X | ||||||||||
| Size and power consumption | X | X | X | X | X | X | |||||
| Usability | X | X | X | ||||||||
| Cost effectiveness | X | X | X | X | X | X | X | ||||
| Incentive facilitation | X | ||||||||||
| Social norms support | X | ||||||||||
| Security and privacy protection | X | X | X | X | X | X |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, T.K.L.; Donyai, P.; McCrindle, R.; Sherratt, R.S. Enabling Medicine Reuse Using a Digital Time Temperature Humidity Sensor in an Internet of Pharmaceutical Things Concept. Sensors 2020, 20, 3080. https://0-doi-org.brum.beds.ac.uk/10.3390/s20113080
Hui TKL, Donyai P, McCrindle R, Sherratt RS. Enabling Medicine Reuse Using a Digital Time Temperature Humidity Sensor in an Internet of Pharmaceutical Things Concept. Sensors. 2020; 20(11):3080. https://0-doi-org.brum.beds.ac.uk/10.3390/s20113080
Chicago/Turabian StyleHui, Terence K. L., Parastou Donyai, Rachel McCrindle, and R. Simon Sherratt. 2020. "Enabling Medicine Reuse Using a Digital Time Temperature Humidity Sensor in an Internet of Pharmaceutical Things Concept" Sensors 20, no. 11: 3080. https://0-doi-org.brum.beds.ac.uk/10.3390/s20113080