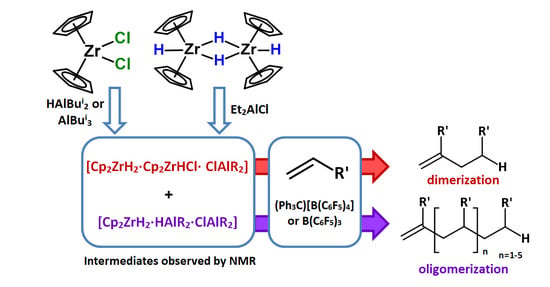
Catalytic Systems Based on Cp2ZrX2 (X = Cl, H), Organoaluminum Compounds and Perfluorophenylboranes: Role of Zr,Zr- and Zr,Al-Hydride Intermediates in Alkene Dimerization and Oligomerization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Study of 1-Hexene Transformations in Cp2ZrCl2-XAlBui2 (X = H, Bui) and [Cp2ZrH2]2-ClAlEt2 Catalytic Systems Modified with Boron Activators
2.2. NMR Study of Hydride Intermediate Structure in the Cp2ZrY2 (Y = H, Cl)-OAC Systems Activated by (Ph3C)[B(C6F5)4] or B(C6F5)3
3. Materials and Methods
3.1. General Procedures
3.2. Reaction of Cp2ZrCl2 with HAlBui2 (AlBui3), (Ph3C)[B(C6F5)4] (B(C6F5)3), and 1-hexne
3.3. Reaction of [Cp2ZrH2]2 with ClAlEt2, (Ph3C)[B(C6F5)4] (B(C6F5)3), and 1-Hexene
3.4. NMR Study of the Reaction of Cp2ZrCl2 with HAlBui2, (Ph3C)[B(C6F5)4], and 1-Hexene
3.5. NMR study of the Reaction of [Cp2ZrH2]2 with ClAlEt2 and (Ph3C)[B(C6F5)4] (B(C6F5)3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collins, R.A.; Russell, A.F.; Mountford, P. Group 4 Metal Complexes for Homogeneous Olefin Polymerisation: A Short Tutorial Review. App. Petrochem. Res. 2015, 5, 153–171. [Google Scholar] [CrossRef] [Green Version]
- Chen, E.Y.-X.; Marks, T.J. Cocatalysts for Metal-Catalyzed Olefin Polymerization: Activators, Activation Processes, and Structure−Activity Relationships. Chem. Rev. 2000, 100, 1391–1434. [Google Scholar] [CrossRef] [PubMed]
- Nifantev, I.; Ivchenko, P.; Tavtorkin, A.; Vinogradov, A.; Vinogradov, A. Non-traditional Ziegler-Natta catalysis in a-olefin transformations: Reaction mechanisms and product design. Pure Appl. Chem. 2017, 89. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Ivchenko, P. Fair Look at Coordination Oligomerization of Higher α-Olefins. Polymers 2020, 12, 1082. [Google Scholar] [CrossRef] [PubMed]
- Hlatky, G.G. Oligomerization & Polymerization by Homogeneous Catalysis. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Janiak, C. Metallocene and Related Catalysts for Olefin, Alkyne and Silane Dimerization and Oligomerization. Coord. Chem. Rev. 2006, 250, 66–94. [Google Scholar] [CrossRef]
- Christoffers, J.; Bergman, R.G. Catalytic Dimerization Reactions of α-Olefins and α,ω-Dienes with Cp2ZrCl2/Poly(methylalumoxane): Formation of Dimers, Carbocycles, and Oligomers. J. Am. Chem. Soc. 1996, 118, 4715–4716. [Google Scholar] [CrossRef]
- Christoffers, J.; Bergman, R.G. Zirconocene-Alumoxane (1:1)—A Catalyst for the Selective Dimerization of α-Olefins. Inor. Chim. Acta 1998, 270, 20–27. [Google Scholar] [CrossRef]
- Baldwin, S.M.; Bercaw, J.E.; Brintzinger, H.H. Alkylaluminum-Complexed Zirconocene Hydrides: Identification of Hydride-Bridged Species by NMR Spectroscopy. J. Am. Chem. Soc. 2008, 130, 17423–17433. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, S.M.; Bercaw, J.E.; Brintzinger, H.H. Cationic Alkylaluminum-Complexed Zirconocene Hydrides as Participants in Olefin Polymerization Catalysis. J. Am. Chem. Soc. 2010, 132, 13969–13971. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, S.M.; Bercaw, J.E.; Henling, L.M.; Day, M.W.; Brintzinger, H.H. Cationic Alkylaluminum-Complexed Zirconocene Hydrides: NMR-Spectroscopic Identification, Crystallographic Structure Determination, and Interconversion with Other Zirconocene Cations. J. Am. Chem. Soc. 2011, 133, 1805–1813. [Google Scholar] [CrossRef] [Green Version]
- Bochmann, M. Highly Electrophilic Organometallics for Carbocationic Polymerizations: From Anion Engineering to New Polymer Materials. Acc. Chem. Res. 2010, 43, 1267–1278. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Ivchenko, P.V. Zirconocene-Catalyzed Dimerization of 1-Hexene: Two-stage Activation and Structure–Catalytic Performance Relationship. Cat. Commun. 2016, 79, 6–10. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Vinogradov, A.; Vinogradov, A.; Karchevsky, S.; Ivchenko, P. Zirconocene-Catalyzed Dimerization of α-Olefins: DFT Modeling of the Zr-Al Binuclear Reaction Mechanism. Molecules 2019, 24, 3565. [Google Scholar] [CrossRef] [Green Version]
- Shamiri, A.; Chakrabarti, M.H.; Jahan, S.; Hussain, M.A.; Kaminsky, W.; Aravind, P.V.; Yehye, W.A. The Influence of Ziegler-Natta and Metallocene Catalysts on Polyolefin Structure, Properties, and Processing Ability. Materials 2014, 7, 5069–5108. [Google Scholar] [CrossRef]
- Soga, K.; Kaminaka, M. Polymerization of Propene with the Heterogeneous Catalyst System Et[IndH4]2ZrCl2/MAO/SiO2 Combined with Trialkylaluminium. Die Makromol. Chem. Rapid Commun. 1992, 13, 221–224. [Google Scholar] [CrossRef]
- Resconi, L.; Piemontesi, F.; Nifant’ev, I.E.; Ivchenko, P.V. Metallocene Compounds, Process for their Preparation, and Their Use in Catalysts for the Polymerization of Olefins. U.S. Patent 6051728, 18 April 2000. [Google Scholar]
- Becke, S.; Rosenthal, U. Aluminoxane Free Catalyst System, Useful for Polymerization of Alpha-olefins, Comprises Fluorine Containing Metal Complex and Trialkyl or Triaryl Boron or Aluminum Compound. U.S. Patent DE19932409A, 18 January 2001. [Google Scholar]
- Becke, S.; Rosenthal, U. Composition Based on Fluorine-Containing Metal Complexes. U.S. Patent 6303718B1, 16 October 2001. [Google Scholar]
- Becke, S.; Rosenthal, U.; Baumann, W.; Arndt, P.; Spannenberg, A. Metallocyclocumulene Compounds Useful as Polymerization Catalysts Are New. U.S. Patent DE10110227A1, 5 September 2002. [Google Scholar]
- Sanginov, E.A.; Panin, A.N.; Saratovskikh, S.L.; Bravaya, N.M. Metallocene Systems in Propylene Polymerization: Effect of Triisobutylaluminum and Lewis Bases on the Behavior of Catalysts and Properties of Polymers. Polym. Sci. Ser. A 2006, 48, 99–106. [Google Scholar] [CrossRef]
- Bravaya, N.M.; Khrushch, N.E.; Babkina, O.N.; Panin, A.N. Formation and Catalytic Properties of Metallocene Systems with Combined Cocatalyst of Al(i-Bu)3 Perfluorophenyl Borate. Ross. Khimicheskij Zhurnal 2001, 45, 56–68. [Google Scholar]
- Bravaya, N.M.; Panin, A.N.; Faingol’d, E.E.; Babkina, O.N.; Razavi, A. C2-symmetry Dimethylated Zirconocenes Activated with Triisobutyl Aluminum as Effective Homogeneous Catalysts for Copolymerization of Olefins. J. Polym. Sci. A Polym. Chem. 2010, 48, 2934–2941. [Google Scholar] [CrossRef]
- Sacco, M.; Nifant’ev, I.; Ivchenko, P.; Bagrov, V.; Focante, F. Metallocene Compounds. U.S. Patent US7803887B2, 28 September 2010. [Google Scholar]
- Nifantev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Sedov, I.V.; Dorokhov, V.G.; Lyadov, A.S.; Ivchenko, P.V. Structurally uniform 1-hexene, 1-octene, and 1-decene oligomers: Zirconocene/MAO-catalyzed preparation, characterization, and prospects of their use as low-viscosity low-temperature oil base stocks. Appl. Cat. A Gen. 2018, 549, 40–50. [Google Scholar] [CrossRef]
- Yang, X.; Stern, C.L.; Marks, T.J. Cationic Metallocene Polymerization Catalysts. Synthesis and Properities of the First Base-Free Zirconocene Hydride. Angew. Chem. Int. Ed. 1992, 31, 1375–1377. [Google Scholar] [CrossRef]
- Yang, X.; Stern, C.L.; Marks, T.J. Cationic Zirconocene Olefin Polymerization Catalysts Based on the Organo-Lewis Acid Tris(pentafluorophenyl)borane. A Synthetic,Structural, Solution Dynamic, and Polymerization Catalytic Study. J. Am. Chem. Soc. 1994, 116, 10015–10031. [Google Scholar] [CrossRef]
- Carr, A.G.; Dawson, D.M.; Thornton-Pett, M.; Bochmann, M. Cationic Zirconocene Hydrides: A New Type of Highly Effective Initiators for Carbocationic Polymerizations. Organometallics 1999, 18, 2933–2935. [Google Scholar] [CrossRef]
- Spence, R.E.V.H.; Parks, D.J.; Piers, W.E.; MacDonald, M.-A.; Zaworotko, M.J.; Rettig, S.J. Competing Pathways in the Reaction of Bis(pentafluorophenyl)borane with Bis(η5-cyclopentadienyl)dimethylzirconium: Methane Elimination versus Methyl–Hydride Exchange and an Example of Pentacoordinate Carbon. Angew. Chem. Int. Ed. 1995, 34, 1230–1233. [Google Scholar] [CrossRef]
- Sun, Y.; Spence, R.E.v.H.; Piers, W.E.; Parvez, M.; Yap, G.P.A. Intramolecular Ion−Ion Interactions in Zwitterionic Metallocene Olefin Polymerization Catalysts Derived from “Tucked-In” Catalyst Precursors and the Highly Electrophilic Boranes XB(C6F5)2 (X = H, C6F5). J. Am. Chem. Soc. 1997, 119, 5132–5143. [Google Scholar] [CrossRef]
- Spence, R.E.v.H.; Piers, W.E.; Sun, Y.; Parvez, M.; MacGillivray, L.R.; Zaworotko, M.J. Mechanistic Aspects of the Reactions of Bis(pentafluorophenyl)borane with the Dialkyl Zirconocenes Cp2ZrR2 (R = CH3, CH2SiMe3, and CH2C6H5). Organometallics 1998, 17, 2459–2469. [Google Scholar] [CrossRef]
- Arndt, P.; Baumann, W.; Spannenberg, A.; Rosenthal, U.; Burlakov, V.V.; Shur, V.B. Reactions of Titanium and Zirconium Derivatives of Bis(trimethylsilyl)acetylene with Tris(pentafluorophenyl)borane: A Titanium(III) Complex of an Alkynylboranate. Angew. Chem. Int. Ed. 2003, 42, 1414–1418. [Google Scholar] [CrossRef]
- Arndt, P.; Jäger-Fiedler, U.; Klahn, M.; Baumann, W.; Spannenberg, A.; Burlakov, V.V.; Rosenthal, U. Formation of Zirconocene Fluoro Complexes: No Deactivation in the Polymerization of Olefins by the Contact-Ion-Pair Catalysts [Cp’2ZrR]+[RB(C6F5)3]−. Angew. Chem. Int. Ed. 2006, 45, 4195–4198. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Talsi, E.P.; Voskoboynikov, A.Z.; Lancaster, S.J.; Bochmann, M. Formation and Structures of Hafnocene Complexes in MAO- and AlBui3/CPh3[B(C6F5)4]-Activated Systems. Organometallics 2008, 27, 6333–6342. [Google Scholar] [CrossRef]
- Joshi, A.; Zijlstra, H.S.; Collins, S.; McIndoe, J.S. Catalyst Deactivation Processes during 1-Hexene Polymerization. ACS Catal. 2020, 10, 7195–7206. [Google Scholar] [CrossRef]
- Götz, C.; Rau, A.; Luft, G. Ternary Metallocene Catalyst Systems Based on Metallocene Dichlorides and AlBu3i/[PhNMe2H][B(C6F5)4]: NMR Investigations of the Influence of Al/Zr Ratios on Alkylation and on Formation of the Precursor of the Active Metallocene Species. J. Mol. Cat. A Chem. 2002, 184, 95–110. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Talsi, E.P.; Semikolenova, N.V.; Zakharov, V.A.; Brand, J.; Alonso-Moreno, C.; Bochmann, M. Formation and Structures of Cationic Zirconium Complexes in Ternary Systems rac-(SBI)ZrX2/AlBu3i/[CPh3][B(C6F5)4] (X=Cl, Me). J. Organomet. Chem. 2007, 692, 859–868. [Google Scholar] [CrossRef] [Green Version]
- Al-Humydi, A.; Garrison, J.C.; Mohammed, M.; Youngs, W.J.; Collins, S. Propene Polymerization Using Ansa-metallocenium Ions: Catalyst Deactivation Processes During Monomer Consumption and Molecular Structures of the Products Formed. Polyhedron 2005, 24, 1234–1249. [Google Scholar] [CrossRef]
- González-Hernández, R.; Chai, J.; Charles, R.; Pérez-Camacho, O.; Kniajanski, S.; Collins, S. Catalytic System for Homogeneous Ethylene Polymerization Based on Aluminohydride−Zirconocene Complexes. Organometallics 2006, 25, 5366–5373. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Khalilov, L.M.; Dzhemilev, U.M. Mechanisms of Reactions of Organoaluminium Compounds with Alkenes and Alkynes Catalyzed by Zr Complexes. Russ. Chem. Rev. 2012, 81, 524–548. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Pechatkina, S.V.; Khalilov, L.M.; Dzhemilev, U.M. Mechanism of Cp2ZrCl2-catalyzed Olefin Hydroalumination by Alkylalanes. Russ. Chem. Bull. 2005, 54, 316–327. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Vil’danova, R.F.; Pechatkina, S.V.; Khalilov, L.M.; Dzhemilev, U.M. New Effective Reagent [Cp2ZrH2·ClAlEt2]2 for Alkene Hydrometallation. J. Organomet. Chem. 2007, 692, 3424–3429. [Google Scholar] [CrossRef]
- Pankratyev, E.Y.; Tyumkina, T.V.; Parfenova, L.V.; Khursan, S.L.; Khalilov, L.M.; Dzhemilev, U.M. DFT and Ab Initio Study on Mechanism of Olefin Hydroalumination by XAlBui2 in the Presence of Cp2ZrCl2 Catalyst. II. Olefin Interaction with Catalytically Active Centers. Organometallics 2011, 30, 6078–6089. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Nifant’ev, I.E.; Khalilov, L.M.; Dzhemilev, U.M. Role of Zr,Al- Hydride Intermediate Structure and Dynamics in Alkene Hydroalumination with XAlBui2 (X = H, Cl, Bui), Catalyzed by Zr η5-Complexes. Organometallics 2015, 34, 3559–3570. [Google Scholar] [CrossRef]
- Pankratyev, E.Y.; Tyumkina, T.V.; Parfenova, L.V.; Khalilov, L.M.; Khursan, S.L.; Dzhemilev, U.M. DFT Study on Mechanism of Olefin Hydroalumination by XAlBui2 in the Presence of Cp2ZrCl2 Catalyst. I. Simulation of Intermediate Formation in Reaction of HAlBui2 with Cp2ZrCl2. Organometallics 2009, 28, 968–977. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Tyumkina, T.V.; Islamov, D.N.; Lyapina, A.R.; Karchevsky, S.G.; Ivchenko, P.V. Reactions of Bimetallic Zr,Al- Hydride Complexes with Methylaluminoxane: NMR and DFT Study. J. Organomet. Chem. 2017, 851, 30–39. [Google Scholar] [CrossRef]
- Parfenova, L.V.; Kovyazin, P.V.; Bikmeeva, A.K. Bimetallic Zr,Zr-Hydride Complexes in Zirconocene Catalyzed Alkene Dimerization. Molecules 2020, 25, 2216. [Google Scholar] [CrossRef] [PubMed]
- Dzhemilev, U.M.; Ibragimov, A.G. Hydrometallation of Unsaturated Compounds; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 447–489. [Google Scholar]
- Dzhemilev, U.M.; Ibragimov, A.G. Metal Complex Catalysis in the Synthesis of Organoaluminium Compounds. Russ. Chem. Rev. 2000, 69, 121–135. [Google Scholar] [CrossRef]
- Shoer, L.I.; Gell, K.I.; Schwartz, J. Mixed-metal Hydride Complexes Containing Zr-H-Al Bridges. Synthesis and Relation to Transition-Metal-Catalyzed Reactions of Aluminum Hydrides. J. Organomet. Chem. 1977, 136, 19–22. [Google Scholar] [CrossRef]
- Claridge, T.D.W. Chapter 8—Correlations through Space: The Nuclear Overhauser Effect; Elsevier: Amsterdam, The Netherlands, 2009; Volume 27, pp. 247–302. [Google Scholar]
- Hassinen, A.; Martins, J.C.; Hens, Z. Solution NMR Toolbox for Colloidal Nanoparticles; Springer: Berlin/Heidelberg, Germany, 2014; pp. 273–293. [Google Scholar] [CrossRef]
- Bochmann, M.; Sarsfield, M.J. Reaction of AlR3 with [CPh3][B(C6F5)4]: Facile Degradation of [B(C6F5)4]- by Transient “[AlR2]+”. Organometallics 1998, 17, 5908–5912. [Google Scholar] [CrossRef]
- Janiak, C.; Lassahn, P.-G. 19F NMR Investigations of the Reaction of B(C6F5)3 with Different Tri(alkyl)aluminum Compounds. Macromol. Symp. 2006, 236, 54–62. [Google Scholar] [CrossRef]
- Chen, H.C.; Chen, S.H. Diffusion of Crown Ethers in Alcohols. J. Phys. Chem. 1984, 88, 5118–5121. [Google Scholar] [CrossRef]
- Zuccaccia, C.; Stahl, N.G.; Macchioni, A.; Chen, M.-C.; Roberts, J.A.; Marks, T.J. NOE and PGSE NMR Spectroscopic Studies of Solution Structure and Aggregation in Metallocenium Ion-Pairs. J. Am. Chem. Soc. 2004, 126, 1448–1464. [Google Scholar] [CrossRef]
- Slaugh, L.H.; Schoenthal, G.W. Vinylidene Olefin Process. U.S. Patent 4658078, 14 April 1987. [Google Scholar]
- Nakata, N.; Nakamura, K.; Ishii, A. Highly Efficient and 1,2-Regioselective Method for the Oligomerization of 1-Hexene Promoted by Zirconium Precatalysts with [OSSO]-Type Bis(phenolate) Ligands. Organometallics 2018, 37, 2640–2644. [Google Scholar] [CrossRef]
- Nakata, N.; Nakamura, K.; Nagaoka, S.; Ishii, A. Carbazolyl-Substituted [OSSO]-Type Zirconium(IV) Complex as a Precatalyst for the Oligomerization and Polymerization of α-Olefins. Catalysts 2019, 9, 528. [Google Scholar] [CrossRef] [Green Version]
- Gunasekara, T.; Preston, A.Z.; Zeng, M.; Abu-Omar, M.M. Highly Regioselective α-Olefin Dimerization Using Zirconium and Hafnium Amine Bis(phenolate) Complexes. Organometallics 2017, 36, 2934–2939. [Google Scholar] [CrossRef]
- Piers, W.E.; Shapiro, P.J.; Bunel, E.E.; Bercaw, J.E. Coping With Extreme Lewis Acidity: Strategies for the Synthesis of Stable, Mononuclear Organometallic Derivatives of Scandium. Synlett 1990, 74–84. [Google Scholar] [CrossRef]
- Kretschmer, W.P.; Troyanov, S.I.; Meetsma, A.; Hessen, B.; Teuben, J.H. Regioselective Homo- and Codimerization of α-Olefins Catalyzed by Bis(2,4,7-trimethylindenyl)yttrium Hydride. Organometallics 1998, 17, 284–286. [Google Scholar] [CrossRef]
- Gibson, V.C.; Kee, T.P.; Poole, A.D. Selective Catalytic Dimerisation of Ethylene to But-1-ene by [(η-C5Me5)Ta(PMe3)(H)(Br)(η2-CHPMe2)]. J. Chem. Soc. Chem. Commun. 1990, 1720–1722. [Google Scholar] [CrossRef]
- Lee, D.W.; Yi, C.S. Chain-Selective and Regioselective Ethylene and Styrene Dimerization Reactions Catalyzed by a Well-Defined Cationic Ruthenium Hydride Complex: New Insights on the Styrene Dimerization Mechanism. Organometallics 2010, 29, 3413–3417. [Google Scholar] [CrossRef] [Green Version]
- McInnis, J.P.; Delferro, M.; Marks, T.J. Multinuclear Group 4 Catalysis: Olefin Polymerization Pathways Modified by Strong Metal–Metal Cooperative Effects. Acc. Chem. Res. 2014, 47, 2545–2557. [Google Scholar] [CrossRef]
- Longsworth, L.G. The Mutual Diffusion of Light and Heavy Water. J. Phys. Chem. 1960, 64, 1914–1917. [Google Scholar] [CrossRef]
- Mills, R. Self-diffusion in Normal and Heavy Water in the Range 1-45.deg. J. Phys. Chem. 1973, 77, 685–688. [Google Scholar] [CrossRef]
- Johnson, C.S. Diffusion Ordered Nuclear Magnetic Resonance Spectroscopy: Principles and Applications. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 34, 203–256. [Google Scholar] [CrossRef]
- Macchioni, G.C.; Zuccaccia, C.; Zuccaccia, D. Diffusion Ordered NMR Spectroscopy (DOSY). Supramol. Chem. 2012. [Google Scholar] [CrossRef]
- Freidlina, R.K.; Brainina, E.M.; Nesmeyanov, A.N. The Synthesis of Mixed Pincerlike Cyclopentadienyl Compounds of Zirconium. Dokl. Acad. Nauk SSSR 1961, 138, 1369–1372. [Google Scholar]











| Entry | Catalytic Systems A or B | [Zr]:[Al]:[B]:[1-alkene] | T, °C | Time, min | Alkene Conversion, % | Product Composition, % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zr Complex | OAC a | Organoboron Activator | 4 | 5 | |||||||||
| n = 1 | n = 2 | n = 3 | n = 4 | n = 5 | |||||||||
| 1 | Cp2ZrCl2 | HAlBui2 | (Ph3C)[B(C6F5)4] | 1:16:1:1000 | 40 | 90 | 60 | 41 | 10 | 4 | 2 | 2 | 1 |
| 2 | 60 | 90 | 95 | 29 | 16 | 15 | 13 | 12 | 9 | ||||
| 3 | HAlBui2 | (Ph3C)[B(C6F5)4] | 4:16:1:1000 | 40 | 90 | 90 | 67 | 10 | 5 | 3 | 2 | 2 | |
| 4 | HAlBui2 | (Ph3C)[B(C6F5)4] | 60 | 90 | 97 | 52 | 14 | 13 | 8 | 7 | 3 | ||
| 5 | AlBui3 | (Ph3C)[B(C6F5)4] | 40 | 90 | 95 | 38 | 14 | 16 | 10 | 9 | 7 | ||
| 6 | HAlBui2 | B(C6F5)3 | 40 | 60 | > 99 | 93 | 3 | - | - | - | - | ||
| 7 | HAlBui2 | (Ph3C)[B(C6F5)4] | 4:25:1:1000 | 40 | 90 | 99 | 52 | 17 | 11 | 9 | 6 | 3 | |
| 8 | HAlBui2 | (Ph3C)[B(C6F5)4] | 4:16:1:400 | 40 | 90 | >99 | 59 | 13 | 10 | 7 | 5 | 5 | |
| 9 | [Cp2ZrH2]2 | ClAlEt2 | (Ph3C)[B(C6F5)4] | 1:3:1:400 | 60 | 150 | 30 | 28 | - | - | - | - | - |
| 10 | ClAlEt2 | (Ph3C)[B(C6F5)4] | 4:8:1:400 | 40 | 150 | 81 | 81 | - | - | - | - | - | |
| 11 | ClAlEt2 | B(C6F5)3 | 40 | 90 | 91 | 86 | - | - | - | - | - | ||
| Complex | δH Cp | δC Cp | δH Zr-H | δH AlR | Dt, 10−10 m2 s−1 | Rh, Å | Vh, Å3 |
|---|---|---|---|---|---|---|---|
| 6a a | 5.61 (s, 10H) | 104.7 | −1.09 (br.t, 6.3 Hz, 1H) −2.28 (br.d, 6.3 Hz, 2H) | 0.32 (m) 1.25 (m) | 9.3 | 4.2 | 319 |
| 7a a | 5.48 (s, 20H) | 107.6 | −1.39 (d, 17.0 Hz, 2H) −6.53 (t, 17.0 Hz, 1H) | 0.17 (q, 8.1 Hz, 4H) 1.35 (t, q, 8.1 Hz, 4H) | 7.5 | 4.9 | 505 |
| 8a a | 5.73 (s, 10H) | 107.2 | −1.66 (br.s, 1H) −2.63 (br.s, 1H) | 0.32 (m) 1.25 (m) | 8.3 | 4.6 | 403 |
| 9a b | 5.30 (s, 20H) | 107.7 | −1.51 (d, 16.8 Hz, 2H) −6.62 (t, 16.8 Hz, 1H) | 0.14 (q, 8.1 Hz, 4H) 1.33 (t, q, 8.1 Hz, 4H) | 5.0 | 6.9 | 1344 |
| 10 b | 5.06 (s, 20H) | 107.5 | −1.72 (br.d, 16.8 Hz, 2H) −6.87 (br.t, 16.8 Hz, 1H) | −0.11 (br.q, 7.2 Hz, 4H) 0.88–1.01 (m) | 1.8 | 17.2 | 21236 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parfenova, L.V.; Kovyazin, P.V.; Bikmeeva, A.K.; Palatov, E.R. Catalytic Systems Based on Cp2ZrX2 (X = Cl, H), Organoaluminum Compounds and Perfluorophenylboranes: Role of Zr,Zr- and Zr,Al-Hydride Intermediates in Alkene Dimerization and Oligomerization. Catalysts 2021, 11, 39. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010039
Parfenova LV, Kovyazin PV, Bikmeeva AK, Palatov ER. Catalytic Systems Based on Cp2ZrX2 (X = Cl, H), Organoaluminum Compounds and Perfluorophenylboranes: Role of Zr,Zr- and Zr,Al-Hydride Intermediates in Alkene Dimerization and Oligomerization. Catalysts. 2021; 11(1):39. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010039
Chicago/Turabian StyleParfenova, Lyudmila V., Pavel V. Kovyazin, Almira Kh. Bikmeeva, and Eldar R. Palatov. 2021. "Catalytic Systems Based on Cp2ZrX2 (X = Cl, H), Organoaluminum Compounds and Perfluorophenylboranes: Role of Zr,Zr- and Zr,Al-Hydride Intermediates in Alkene Dimerization and Oligomerization" Catalysts 11, no. 1: 39. https://0-doi-org.brum.beds.ac.uk/10.3390/catal11010039