Maternal Melatonin Therapy Attenuates Methyl-Donor Diet-Induced Programmed Hypertension in Male Adult Rat Offspring
Abstract
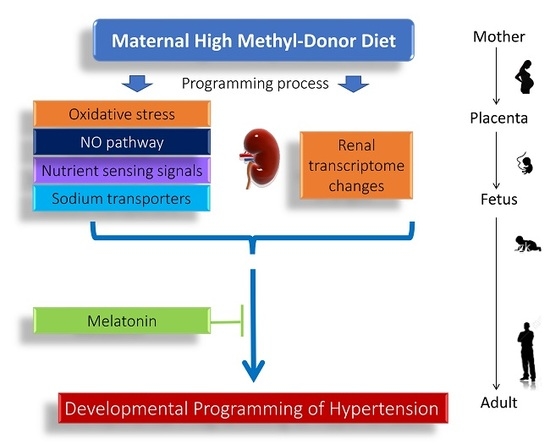
:1. Introduction
2. Materials and Methods
2.1. Animal Study
2.2. Quantitative Real-Time Polymerase Chain Reaction (qPCR)
2.3. High-Performance Liquid Chromatography (HPLC)
2.4. Next-Generation Sequencing and Analysis
2.5. Immunohistochemistry Staining
2.6. Statistical Analysis
3. Results
3.1. Morphological Features, Biochemical Values, and Blood Pressure
3.2. Renal Transcriptome
3.3. Nutrient-Sensing Pathway and Sodium Transporters
3.4. ADMA-NO Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Langley-Evans, S.C. Nutritional programming of disease: Unravelling the mechanism. J. Anat. 2009, 215, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.; Gluckman, P. Developmental origins of noncommunicable disease: Population and public health implications. Am. J. Clin. Nutr. 2011, 94, 1754S–1758S. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wahlqvist, M.L.; Li, D. Nutrition, One-Carbon Metabolism and Neural Tube Defects: A Review. Nutrients 2016, 8, 741. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, R.J.; Vrana, P.B. Rosenfeld CS. Maternal methyl supplemented diets and effects on offspring health. Front. Genet. 2014, 5. [Google Scholar] [CrossRef]
- Bianco-Miotto, T.; Craig, J.M.; Gasser, Y.P.; van Dijk, S.J.; Ozanne, S.E. Epigenetics and DOHaD: From basics to birth and beyond. J. Dev. Orig. Health Dis. 2017, 8, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.S.; Power, B.E.; Molloy, P.L. DNA hypomethylation and human diseases. Biochim. Biophys. Acta 2007, 1775, 138–162. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins (Basel) 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.T.; Hsieh, C.S.; Chang, K.A.; Tain, Y.L. Roles of nitric oxide and asymmetric dimethylarginine in pregnancy and fetal programming. Int. J. Mol. Sci. 2012, 13, 14606–14622. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Targeting on Asymmetric Dimethylarginine-Related Nitric Oxide-Reactive Oxygen Species Imbalance to Reprogram the Development of Hypertension. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, K.; Li, X.; Datta, J.; Bai, S.; Pogribny, I.; Pogribny, M.; Huang, Y.; Young, D.; Jacob, S.T. A folate- and methyl-deficient diet alters the expression of DNA methyltransferases and methyl CpG binding proteins involved in epigenetic gene silencing in livers of F344 rats. J. Nutr. 2006, 136, 1522–1527. [Google Scholar] [CrossRef] [PubMed]
- Maloney, C.A.; Hay, S.M.; Young, L.E.; Sinclair, K.D.; Rees, W.D. A methyl-deficient diet fed to rat dams during the peri-conception period programs glucose homeostasis in adult male but not female offspring. J. Nutr. 2011, 141, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Dolinoy, D.C.; Huang, D.; Jirtle, R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. USA 2007, 104, 13056–13061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giudicelli, F.; Brabant, A.L.; Grit, I.; Parnet, P.; Amarger, V. Excess of methyl donor in the perinatal period reduces postnatal leptin secretion in rat and interacts with the effect of protein content in diet. PLoS ONE 2013, 8, e68268. [Google Scholar] [CrossRef] [PubMed]
- Shorter, K.R.; Anderson, V.; Cakora, P.; Owen, A.; Lo, K.; Crossland, J.; South, A.C.; Felder, M.R.; Vrana, P.B. Pleiotropic effects of a methyl donor diet in a novel animal model. PLoS ONE 2014, 9, e104942. [Google Scholar] [CrossRef] [PubMed]
- Schaible, T.D.; Harris, R.A.; Dowd, S.E.; Smith, C.W.; Kellermayer, R. Maternal methyl-donor supplementation induces prolonged murine offspring colitis susceptibility in association with mucosal epigenetic and microbiomic changes. Hum. Mol. Genet. 2011, 20, 1687–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tain, Y.L.; Hsu, C.N.; Chan, J.Y.; Huang, L.T. Renal Transcriptome Analysis of Programmed Hypertension Induced by Maternal Nutritional Insults. Int. J. Mol. Sci. 2015, 16, 17826–17837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kett, M.M.; Denton, K.M. Renal programming: Cause for concern? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R791–R803. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Joles, J.A. Reprogramming: A preventive strategy in hypertension focusing on the kidney. Int. J. Mol. Sci. 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Huang, L.T.; Hsu, C.N. Developmental Programming of Adult Disease: Reprogramming by Melatonin? Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Huang, L.T.; Chan, J.Y. Transcriptional regulation of programmed hypertension by melatonin: An epigenetic perspective. Int. J. Mol. Sci. 2014, 15, 18484–18495. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, A.; Reiter, R.J. Epigenetic regulation: A new research area for melatonin? J. Pineal Res. 2008, 44, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Huang, L.T.; Hsu, C.N.; Lee, C.T. Melatonin therapy prevents programmed hypertension and nitric oxide deficiency in offspring exposed to maternal caloric restriction. Oxid. Med. Cell Longev. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Lee, W.C.; Wu, K.; Leu, S.; Chan, J.Y.H. Resveratrol prevents the development of hypertension programmed by maternal plus post-weaning high-fructose consumption through modulation of oxidative stress, nutrient-sensing signals, and gut microbiota. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef] [PubMed]
- Cordero, P.; Campion, J.; Milagro, F.I.; Martinez, J.A. Transcriptomic and epigenetic changes in early liver steatosis associated to obesity: Effect of dietary methyl donor supplementation. Mol. Genet. Metab. 2013, 110, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F. Gender differences in the regulation of blood pressure. Hypertension 2011, 37, 1199–1208. [Google Scholar] [CrossRef]
- Tain, Y.L.; Freshour, G.; Dikalova, A.; Griendling, K.; Baylis, C. Vitamin E reduces glomerulosclerosis, restores renal neuronal NOS, and suppresses oxidative stress in the 5/6 nephrectomized rat. Am. J. Physiol. Ren. Physiol. 2007, 292, F1404–F1410. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.J.; Lin, I.C.; Hsu, C.N.; Lo, M.H.; Tain, Y.L. Homocysteine and Arginine-to-Asymmetric Dimethylarginine Ratio Associated with Blood Pressure Abnormalities in Children with Early Chronic Kidney Disease. Circ. J. 2015, 79, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Dennis, G., Jr.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4. [Google Scholar] [CrossRef] [Green Version]
- Jansson, T.; Powell, T.L. Role of placental nutrient sensing in developmental programming. Clin. Obstet. Gynecol. 2013, 56, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N. Interplay between oxidative stress and nutrient sensing signaling in the developmental origins of cardiovascular disease. Int. J. Mol. Sci. 2017, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.S.; Zhou, Y.M.; Li, D.; Lun, Y.Z. Dietary methyl-consuming compounds and metabolic syndrome. Hypertens. Res. 2011, 34, 1239–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinclair, K.D.; Allegrucci, C.; Singh, R.; Gardner, D.S.; Sebastian, S.; Bispham, J.; Thurston, A.; Huntley, J.F.; Rees, W.D.; Maloney, C.A.; et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc. Natl. Acad. Sci. USA 2007, 104, 19351–19356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Veyver, I.B. Genetic effects of methylation diets. Annu. Rev. Nutr. 2002, 22, 255–282. [Google Scholar] [CrossRef] [PubMed]
- Buffat, C.; Boubred, F.; Mondon, F.; Chelbi, S.T.; Feuerstein, J.M.; Lelièvre-Pégorier, M.; Vaiman, D.; Simeoni, U. Kidney gene expression analysis in a rat model of intrauterine growth restriction reveals massive alterations of coagulation genes. Endocrinology 2007, 148, 5549–5557. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Wu, K.L.; Lee, W.C.; Leu, S.; Chan, J.Y. Maternal fructose-intake-induced renal programming in adult male offspring. J. Nutr. Biochem. 2015, 26, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Ajith, T.A.; Jayakumar, T.G. Peroxisome proliferator-activated receptors in cardiac energy metabolism and cardiovascular disease. Clin. Exp. Pharmacol. Physiol. 2016, 43, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Hsu, C.N.; Chan, J.Y. PPARs Link Early Life Nutritional Insults to Later Programmed Hypertension and Metabolic Syndrome. Int. J. Mol. Sci. 2015, 17. [Google Scholar] [CrossRef] [PubMed]
- Usuda, D.; Kanda, T. Peroxisome proliferator-activated receptors for hypertension. World J. Cardiol. 2014, 6, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.H.; Kuo, H.C.; Lin, I.C.; Chien, S.J.; Huang, L.T.; Tain, Y.L. Melatonin prevents neonatal dexamethasone induced programmed hypertension: Histone deacetylase inhibition. J. Steroid Biochem. Mol. Biol. 2014, 144, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, G.; Marr, M.; Myers, C.; Wilson, R.; Travlos, G.; Price, C. Maternal and developmental toxicity evaluation of melatonin administered orally to pregnant Sprague-Dawley rats. Toxicol. Sci. 1999, 50, 271–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Huang, L.T.; Chan, J.Y.; Lee, C.T. Transcriptome analysis in rat kidneys: Importance of genes involved in programmed hypertension. Int. J. Mol. Sci. 2015, 16, 4744–4758. [Google Scholar] [CrossRef] [PubMed]




| Gene | Forward | Reverse |
|---|---|---|
| Sirt1 | 5′-TGGAGCAGGTTGCAGGAATCCA-3′ | 5′-TGGCTTCATGATGGCAAGTGGC-3′ |
| Ppara | 5′-AGAAGTTGCAGGAGGGGATT-3′ | 5′-TTCTTGATGACCTGCACGAG-3′ |
| Pparrb | 5′-GATCAGCGTGCATGTGTTCT-3′ | 5′-CAGCAGTCCGTCTTTGTTGA-3′ |
| Pparg | 5′-CTTTATGGAGCCTAAGTTTGAGT-3′ | 5′-GTTGTCTTGGATGTCCTCG-3′ |
| Pargc1ap | 5′-CCCATTGAGGGCTGTGATCT-3′ | 5′-TCAGTGAAATGCCGGAGTCA-3′ |
| Prkaa2 | 5′-AGCTCGCAGTGGCTTATCAT-3′ | 5′-GGGGCTGTCTGCTATGAGAG-3′ |
| Prkab2 | 5′-CAGGGCCTTATGGTCAAGAA-3′ | 5′-CAGCGCATAGAGATGGTTCA-3′ |
| Prkag2 | 5′-GTGTGGGAGAAGCTCTGAGG-3′ | 5′-AGACCACACCCAGAAGATGC-3′ |
| Slc9a3 | 5′-CATTTGTCCCTTTCCGAATTG-3′ | 5′-CCAAATGGCAGCTCCAAATAG-3′ |
| Slc12a1 | 5′-ACAGGAGGACCCATGACAAGA-3′ | 5′-GCAGCAGATACAGAGGCCACTA-3′ |
| Slc12a3 | 5′-TGATCCGATGCATGCTCAA-3′ | 5′-CGCCTGCGCCGTAATC-3′ |
| Atp1a1 | 5′-GGCTGTCATCTTCCTCATTGG-3′ | 5′-CGGTGGCCAGCAAACC-3′ |
| Rn18s | 5′-GCCGCGGTAATTCCAGCTCCA-3′ | 5′-CCCGCCCGCTCCCAAGATC-3′ |
| Parameter | Control | L-MD | H-MD | H-MD+M |
|---|---|---|---|---|
| n = 8 | n = 8 | n = 7 | n = 8 | |
| Body weight (BW) (g) | 378 ± 9 | 405 ± 12 | 352 ± 9 b | 411 ± 7 c |
| Left kidney weight (g) | 1.39 ± 0.06 | 1.80 ± 0.08 | 1.60 ± 0.05 | 1.60 ± 0.05 |
| Left kidney weight/ 100 g BW | 0.49 ± 0.01 | 0.46 ± 0.01 | 0.46 ± 0.01 | 0.40 ± 0.01 a,b,c |
| Systolic blood pressure (mmHg) | 136 ± 1 | 150 ± 1 a | 148 ± 1 a | 141 ± 1 b,c |
| Creatinine (μM) | 17.6 ± 0.6 | 16.7 ± 0.7 | 19.8 ± 0.9 | 18.4 ± 0.5 |
| Items | Count | % | p-Value | Benjamini |
|---|---|---|---|---|
| L-MD: 10 KEGG pathways | ||||
| Ribosome | 23 | 4.4 | 4.3 × 10−11 | 9.1 × 10−9 |
| Complement and coagulation cascades | 9 | 1.7 | 2.3 × 10−4 | 2.4 × 10−2 |
| Staphylococcus aureus infection | 7 | 1.4 | 1.4 × 10−3 | 9.5 × 10−2 |
| PPAR signaling pathway | 7 | 1.4 | 8.4 × 10−3 | 3.6 × 10−1 |
| African trypanosomiasis | 5 | 1.0 | 1.1 × 10−2 | 3.7 × 10−1 |
| Phagosome | 11 | 2.1 | 1.5 × 10−2 | 4.1 × 10−1 |
| Herpes simplex infection | 11 | 2.1 | 2.8 × 10−2 | 5.7 × 10−1 |
| Transcriptional misregulation in cancer | 9 | 1.7 | 3.3 × 10−2 | 5.9 × 10−1 |
| Malaria | 5 | 1.0 | 4.6 × 10−2 | 6.7 × 10−1 |
| Type I diabetes mellitus | 5 | 1.0 | 9.7 × 10−2 | 8.9 × 10−1 |
| H-MD: 21 KEGG pathways | ||||
| Complement and coagulation cascades | 12 | 1.7 | 2.2 × 10−5 | 5.3 × 10−3 |
| Choline metabolism in cancer | 12 | 1.7 | 5.0 × 10−4 | 5.8 × 10−2 |
| FoxO signaling pathway | 11 | 1.6 | 1.5 × 10−2 | 7.0 × 10−1 |
| Proteoglycans in cancer | 14 | 2.0 | 1.7 × 10−2 | 6.3 × 10−1 |
| Influenza A | 12 | 1.7 | 2.7 × 10−2 | 7.3 × 10−1 |
| Herpes simplex infection | 14 | 2.0 | 2.8 × 10−2 | 6.7 × 10−1 |
| TNF signaling pathway | 9 | 1.3 | 2.9 × 10−2 | 6.3 × 10−1 |
| Type II diabetes mellitus | 6 | 0.9 | 2.9 × 10−2 | 5.8 × 10−1 |
| Staphylococcus aureus infection | 6 | 0.9 | 3.3 × 10−2 | 5.9 × 10−1 |
| Type I diabetes mellitus | 7 | 1.0 | 4.0 × 10−2 | 6.2 × 10−1 |
| Arachidonic acid metabolism | 7 | 1.0 | 5.2 × 10−2 | 6.9 × 10−1 |
| Cytokine-cytokine receptor interaction | 13 | 1.9 | 5.7 × 10−2 | 6.8 × 10−1 |
| Maturity onset diabetes of the young | 4 | 0.6 | 5.9 × 10−2 | 6.7 × 10−1 |
| HTLV-I infection | 16 | 2.3 | 6.1 × 10−2 | 6.6 × 10−1 |
| Graft-versus-host disease | 6 | 0.9 | 6.8 × 10−2 | 6.7 × 10−1 |
| Pathways in cancer | 20 | 2.9 | 7.1 × 10−2 | 6.7 × 10−1 |
| Prostate cancer | 7 | 1.0 | 7.2 × 10−2 | 6.5 × 10−1 |
| Tuberculosis | 11 | 1.6 | 8.2 × 10−2 | 6.8 × 10−1 |
| ErbB signaling pathway | 7 | 1.0 | 8.2 × 10−2 | 6.6 × 10−1 |
| Allograft rejection | 6 | 0.9 | 8.3 × 10−2 | 6.4 × 10−1 |
| Bile secretion | 6 | 0.9 | 8.7 × 10−2 | 6.4 × 10−1 |
| H-MD+M: 8 KEGG pathways | ||||
| Complement and coagulation cascades | 9 | 1.5 | 5.7 × 10−4 | 1.2 × 10−1 |
| PPAR signaling pathway | 7 | 1.2 | 1.6 × 10−2 | 8.2 × 10−1 |
| Metabolic pathways | 47 | 7.9 | 1.7 × 10−2 | 7.2 × 10−1 |
| Bile secretion | 6 | 1.0 | 3.9 × 10−2 | 8.8 × 10−1 |
| Biosynthesis of unsaturated fatty acids | 4 | 0.7 | 4.0 × 10−2 | 8.3 × 10−1 |
| Staphylococcus aureus infection | 5 | 0.8 | 5.3 × 10−2 | 8.6 × 10−1 |
| TNF signaling pathway | 7 | 1.2 | 6.8 × 10−2 | 8.8 × 10−1 |
| Phagosome | 10 | 1.7 | 7.6 × 10−2 | 8.8 × 10−1 |
| Parameter | Control | L-MD | H-MD | H-MD+M |
|---|---|---|---|---|
| l-Citrulline (μM) | 106 ± 7 | 110 ± 8 | 118 ± 14 | 108 ± 13 |
| l-Arginine (μM) | 341 ± 18 | 371 ± 32 | 404 ± 25 | 334 ± 20 |
| ADMA (μM) | 1.88 ± 0.26 | 1.8 ± 0.18 | 1.81 ± 0.19 | 1.56 ± 0.09 |
| SDMA (μM) | 3.01 ± 0.35 | 4.21 ± 0.17 a | 3.23 ± 0.37 | 2.89 ± 0.25 b |
| (ADMA+SDMA)-to-l-arginine ratio (μM/μM) | 0.014 ± 0.002 | 0.017 ± 0.001 | 0.012 ± 0.001 | 0.015 ± 0.002 |
| Homocysteine (μM) | 3.84 ± 0.41 | 3.67 ± 0.58 | 4.81 ± 0.48 | 3.13 ± 0.42 |
| Urine NO2− (μM/24 h/kg BW) | 0.42 ± 0.07 | 0.32 ± 0.03 | 0.43 ± 0.15 | 0.55 ± 0.04 b |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tain, Y.-L.; Chan, J.Y.H.; Lee, C.-T.; Hsu, C.-N. Maternal Melatonin Therapy Attenuates Methyl-Donor Diet-Induced Programmed Hypertension in Male Adult Rat Offspring. Nutrients 2018, 10, 1407. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10101407
Tain Y-L, Chan JYH, Lee C-T, Hsu C-N. Maternal Melatonin Therapy Attenuates Methyl-Donor Diet-Induced Programmed Hypertension in Male Adult Rat Offspring. Nutrients. 2018; 10(10):1407. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10101407
Chicago/Turabian StyleTain, You-Lin, Julie Y. H. Chan, Chien-Te Lee, and Chien-Ning Hsu. 2018. "Maternal Melatonin Therapy Attenuates Methyl-Donor Diet-Induced Programmed Hypertension in Male Adult Rat Offspring" Nutrients 10, no. 10: 1407. https://0-doi-org.brum.beds.ac.uk/10.3390/nu10101407