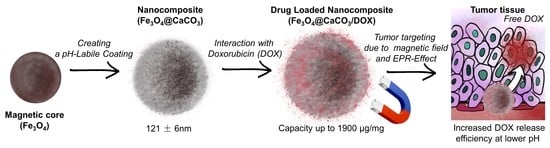
pH-Responsible Doxorubicin-Loaded Fe3O4@CaCO3 Nanocomposites for Cancer Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of NPs
2.3. Fe3O4@CaCO3 Nanocomposite Synthesis
2.4. Fe3O4@CaCO3 Stability
2.5. Doxorubicin-Loaded Fe3O4@CaCO3 Synthesis
2.6. Doxorubicin Release from Fe3O4@CaCO3
2.7. Human Serum Albumin and Fe3O4@CaCO3/DOX Interactions
2.8. The Cytotoxicity Assay (MTT Test)
3. Results and Discussion
3.1. Synthesis and Characterization of Fe3O4@CaCO3
3.2. Anticancer Drug Doxorubicin (DOX) Loading
3.3. Doxorubicin Release
3.4. Cellular Toxicity Study of Fe3O4@CaCO3 and Fe3O4@CaCO3/DOX
3.5. Human Serum Albumin Interaction with Fe3O4@CaCO3/DOX
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popova, V.; Dmitrienko, E.; Chubarov, A. Magnetic Nanocomposites and Imprinted Polymers for Biomedical Applications of Nucleic Acids. Magnetochemistry 2023, 9, 12. [Google Scholar] [CrossRef]
- Petrov, K.D.; Chubarov, A.S. Magnetite Nanoparticles for Biomedical Applications. Encyclopedia 2022, 2, 1811–1828. [Google Scholar] [CrossRef]
- Chubarov, A.S. Serum Albumin for Magnetic Nanoparticles Coating. Magnetochemistry 2022, 8, 13. [Google Scholar] [CrossRef]
- Anderson, S.D.; Gwenin, V.V.; Gwenin, C.D. Magnetic Functionalized Nanoparticles for Biomedical, Drug Delivery and Imaging Applications. Nanoscale Res. Lett. 2019, 14, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamichhane, N.; Sharma, S.; Parul; Verma, A.K.; Roy, I.; Sen, T. Iron Oxide-Based Magneto-Optical Nanocomposites for in Vivo Biomedical Applications. Biomedicines 2021, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Creţu, B.E.B.; Dodi, G.; Shavandi, A.; Gardikiotis, I.; Şerban, I.L.; Balan, V. Imaging Constructs: The Rise of Iron Oxide Nanoparticles. Molecules 2021, 26, 3437. [Google Scholar] [CrossRef] [PubMed]
- Anik, M.I.; Hossain, M.K.; Hossain, I.; Mahfuz, A.M.U.B.; Rahman, M.T.; Ahmed, I. Recent Progress of Magnetic Nanoparticles in Biomedical Applications: A Review. Nano Sel. 2021, 2, 1146–1186. [Google Scholar] [CrossRef]
- Mittal, A.; Gandhi, S.; Roy, I. Mechanistic Interaction Studies of Synthesized ZIF-8 Nanoparticles with Bovine Serum Albumin Using Spectroscopic and Molecular Docking Approaches. Sci. Rep. 2022, 12, 10331. [Google Scholar] [CrossRef]
- Comanescu, C. Magnetic Nanoparticles: Current Advances in Nanomedicine, Drug Delivery and MRI. Chemistry 2022, 4, 872–930. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Gorgoń, S.; Radoń, A.; Bajdak-Rusinek, K. Magnetite Nanoparticles in Magnetic Hyperthermia and Cancer Therapies: Challenges and Perspectives. Nanomaterials 2022, 12, 1807. [Google Scholar] [CrossRef]
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.S.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic Nanoparticles in Biomedical Applications: A Review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Mittal, A.; Roy, I.; Gandhi, S. Magnetic Nanoparticles: An Overview for Biomedical Applications. Magnetochemistry 2022, 8, 107. [Google Scholar] [CrossRef]
- Krishnan, S.; Goud, K.Y. Magnetic Particle Bioconjugates: A Versatile Sensor Approach. Magnetochemistry 2019, 5, 64. [Google Scholar] [CrossRef] [Green Version]
- Frenea-Robin, M.; Marchalot, J. Basic Principles and Recent Advances in Magnetic Cell Separation. Magnetochemistry 2022, 8, 11. [Google Scholar] [CrossRef]
- Mariño, M.A.; Fulaz, S.; Tasic, L. Magnetic Nanomaterials as Biocatalyst Carriers for Biomass Processing: Immobilization Strategies, Reusability, and Applications. Magnetochemistry 2021, 7, 133. [Google Scholar] [CrossRef]
- Chouhan, R.S.; Horvat, M.; Ahmed, J.; Alhokbany, N.; Alshehri, S.M.; Gandhi, S. Magnetic Nanoparticles—A Multifunctional Potential Agent for Diagnosis and Therapy. Cancers 2021, 13, 2213. [Google Scholar] [CrossRef] [PubMed]
- Bobrikova, E.; Chubarov, A.; Dmitrienko, E. The Effect of PH and Buffer on Oligonucleotide Affinity for Iron Oxide Nanoparticles. Magnetochemistry 2021, 7, 128. [Google Scholar] [CrossRef]
- Bulgakova, A.; Chubarov, A.; Dmitrienko, E. Magnetic Nylon 6 Nanocomposites for the Microextraction of Nucleic Acids from Biological Samples. Magnetochemistry 2022, 8, 85. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, C.; Liu, S.; Lu, C.; Liu, D.; Pan, Y.; Sakiyama, H.; Muddassir, M.; Liu, J. A New Magnetic Adsorbent of Eggshell-Zeolitic Imidazolate Framework for Highly Efficient Removal of Norfloxacin. Dalt. Trans. 2021, 50, 18016–18026. [Google Scholar] [CrossRef]
- Zheng, R.; Guo, J.; Cai, X.; Bin, L.; Lu, C.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Manganese Complexes and Manganese-Based Metal-Organic Frameworks as Contrast Agents in MRI and Chemotherapeutics Agents: Applications and Prospects. Colloids Surf. B Biointerfaces 2022, 213, 112432. [Google Scholar] [CrossRef]
- Li, C.; Chen, T.; Ocsoy, I.; Zhu, G.; Yasun, E.; You, M.; Wu, C.; Zheng, J.; Song, E.; Huang, C.Z.; et al. Gold-Coated Fe3O4 Nanoroses with Five Unique Functions for Cancer Cell Targeting, Imaging, and Therapy. Adv. Funct. Mater. 2014, 24, 1772–1780. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Wu, L.; Liu, J.; Xie, M.; Shen, H.; Qi, X.; Yan, Y.; Ge, Y.; Jin, Y. Core-Shell Structured Fe3O4@TiO2-Doxorubicin Nanoparticles for Targeted Chemo-Sonodynamic Therapy of Cancer. Int. J. Pharm. 2015, 486, 380–388. [Google Scholar] [CrossRef]
- Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Melnikov, M.Y. Magnetic Nanoparticles for Biomedical Purposes: Modern Trends and Prospects. Magnetochemistry 2020, 6, 30. [Google Scholar] [CrossRef]
- Ganapathe, L.S.; Mohamed, M.A.; Yunus, R.M.; Berhanuddin, D.D. Magnetite (Fe3O4) Nanoparticles in Biomedical Application: From Synthesis to Surface Functionalisation. Magnetochemistry 2020, 6, 68. [Google Scholar] [CrossRef]
- Hepel, M. Magnetic Nanoparticles for Nanomedicine. Magnetochemistry 2020, 6, 3. [Google Scholar] [CrossRef] [Green Version]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic Iron Oxide Nanoparticles-Current and Prospective Medical Applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stueber, D.D.; Villanova, J.; Aponte, I.; Xiao, Z. Magnetic Nanoparticles in Biology and Medicine: Past, Present, and Future Trends. Pharmaceutics 2021, 13, 943. [Google Scholar] [CrossRef] [PubMed]
- Socoliuc, V.; Peddis, D.; Petrenko, V.I.; Avdeev, M.V.; Susan-Resiga, D.; Szabó, T.; Turcu, R.; Tombácz, E.; Vékás, L. Magnetic Nanoparticle Systems for Nanomedicine—A Materials Science Perspective. Magnetochemistry 2020, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Bruschi, M.L.; de Toledo, L.D.A.S. Pharmaceutical Applications of Iron-Oxide Magnetic Nanoparticles. Magnetochemistry 2019, 5, 50. [Google Scholar] [CrossRef] [Green Version]
- Ajalli, N.; Pourmadadi, M.; Yazdian, F.; Rashedi, H.; Navaei-Nigjeh, M.; Díez-Pascual, A.M. Chitosan/Gamma-Alumina/Fe3O4@5-FU Nanostructures as Promising Nanocarriers: Physiochemical Characterization and Toxicity Activity. Molecules 2022, 27, 5369. [Google Scholar] [CrossRef]
- Schubert, J.; Chanana, M. Coating Matters: Review on Colloidal Stability of Nanoparticles with Biocompatible Coatings in Biological Media, Living Cells and Organisms. Curr. Med. Chem. 2018, 25, 4553–4586. [Google Scholar] [CrossRef] [PubMed]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic Nanoparticles: Surface Effects and Properties Related to Biomedicine Applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef] [Green Version]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef]
- Schwaminger, S.P.; Bauer, D.; Fraga-García, P. Gold-Iron Oxide Nanohybrids: Insights into Colloidal Stability and Surface-Enhanced Raman Detection. Nanoscale Adv. 2021, 3, 6438–6445. [Google Scholar] [CrossRef] [PubMed]
- Mourdikoudis, S.; Kostopoulou, A.; LaGrow, A.P. Magnetic Nanoparticle Composites: Synergistic Effects and Applications. Adv. Sci. 2021, 8, 2004951. [Google Scholar] [CrossRef]
- Zou, H.; Luo, Z.; Yang, X.; Xie, Q.; Zhou, Y. Toward Emerging Applications Using Core–Shell Nanostructured Materials: A Review. J. Mater. Sci. 2022, 57, 10912–10942. [Google Scholar] [CrossRef]
- Dinc, M.; Esen, C.; Mizaikoff, B. Recent Advances on Core–Shell Magnetic Molecularly Imprinted Polymers for Biomacromolecules. Trends Anal. Chem. 2019, 114, 202–217. [Google Scholar] [CrossRef]
- Gopalan Sibi, M.; Verma, D.; Kim, J. Magnetic Core–Shell Nanocatalysts: Promising Versatile Catalysts for Organic and Photocatalytic Reactions. Catal. Rev. Sci. Eng. 2020, 62, 163–311. [Google Scholar] [CrossRef]
- Trofimov, A.D.; Ivanova, A.A.; Zyuzin, M.V.; Timin, A.S. Porous Inorganic Carriers Based on Silica, Calcium Carbonate and Calcium Phosphate for Controlled/Modulated Drug Delivery: Fresh Outlook and Future Perspectives. Pharmaceutics 2018, 10, 167. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Tian, Y.; You, J.; Hu, X. Recent Advances of Calcium Carbonate Nanoparticles for Biomedical Applications. Bioengineering 2022, 9, 691. [Google Scholar] [CrossRef]
- Popova, V.; Poletaeva, Y.; Pyshnaya, I.; Pyshnyi, D.; Dmitrienko, E. Designing PH-Dependent Systems Based on Nanoscale Calcium Carbonate for the Delivery of an Antitumor Drug. Nanomaterials 2021, 11, 2794. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Hua, J.; Xie, X. Polyethylenimine-CO2 Adduct-Stabilized Vaterite Hydrocolloidal Particles. Mater. Chem. Phys. 2023, 294, 127025. [Google Scholar] [CrossRef]
- Persano, F.; Nobile, C.; Piccirillo, C.; Gigli, G.; Leporatti, S. Monodisperse and Nanometric-Sized Calcium Carbonate Particles Synthesis Optimization. Nanomaterials 2022, 12, 1494. [Google Scholar] [CrossRef]
- Atchudan, R.; Perumal, S.; Joo, J.; Lee, Y.R. Synthesis and Characterization of Monodispersed Spherical Calcium Oxide and Calcium Carbonate Nanoparticles via Simple Pyrolysis. Nanomaterials 2022, 12, 2424. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Feng, L.; Zhu, W.; Sun, X.; Gao, M.; Zhao, H.; Chao, Y.; Liu, Z. CaCO3 Nanoparticles as an Ultra-Sensitive Tumor-PH-Responsive Nanoplatform Enabling Real-Time Drug Release Monitoring and Cancer Combination Therapy. Biomaterials 2016, 110, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Q.; Li, J.; Sun, X.; Shen, J.; Han, W.; Wang, L. The Preparation of Layered Hierarchical and Cube-Shaped Magnetic Fe3O4/CaCO3 for Efficient Enrichment of Pb(Ⅱ) from Aqueous Solutions. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100600. [Google Scholar] [CrossRef]
- Wang, P.; Xue, J.; Wu, S.; Pei, Y.; Xu, L.; Wang, Y. Cell-Friendly Isolation and PH-Sensitive Controllable Release of Circulating Tumor Cells by Fe3O4@CaCO3 Nanoplatform. Adv. Mater. Interfaces 2021, 8, 2101191. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Sharifi, S.; Ahmadian, E.; Eftekhari, A.; Adibkia, K.; Lotfipour, F. An Update on Calcium Carbonate Nanoparticles as Cancer Drug/Gene Delivery System. Expert Opin. Drug Deliv. 2019, 16, 331–345. [Google Scholar] [CrossRef]
- Vavaev, E.S.; Novoselova, M.; Shchelkunov, N.M.; German, S.; Aleksei, S.; Mokrousov, M.D.; Zelepukin, I.V.; Burov, A.M.; Khlebtsov, B.N.; Lyubin, E.V.; et al. CaCO3 Nanoparticles Coated with Alternating Layers of Poly-L-Arginine Hydrochloride and Fe3O4 Nanoparticles as Navigable Drug Carriers and Hyperthermia Agents. ACS Appl. Nano Mater. 2022, 5, 2994–3006. [Google Scholar] [CrossRef]
- Xue, J.; Li, X.; Li, Q.; Lyu, J.; Wang, W.; Zhuang, L.; Xu, Y. Magnetic Drug-Loaded Osteoinductive Fe3O4/CaCO3 Hybrid Microspheres System: Efficient for Sustained Release of Antibiotics. J. Phys. D Appl. Phys. 2020, 53, 245401. [Google Scholar] [CrossRef]
- Serov, N.; Prilepskii, A.; Sokolov, A.; Vinogradov, V. Synthesis of Plasmin-Loaded Fe3O4@CaCO3 Nanoparticles: Towards Next-Generation Thrombolytic Drugs. ChemNanoMat 2019, 5, 1267–1271. [Google Scholar] [CrossRef]
- Li, F.H.; Tang, N.; Wang, Y.Q.; Zhang, L.; Du, W.; Xiang, J.; Cheng, P.G. Synthesis and Characterization of Magnetic Carriers Based on Immobilized Enzyme. IOP Conf. Ser. Mater. Sci. Eng. 2018, 359, 012044. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Seo, J.C.; Oh, Y.K.; Lee, K. Synthesis of Microaglae-Capturing Magnetic Microcapsule Using CaCO3 Microparticles and Layer-by-Layer Coating. Korean J. Mater. Res. 2018, 28, 376–380. [Google Scholar] [CrossRef]
- Han, P.; Jiang, Z.; Wang, X.; Wang, X.; Zhang, S.; Shi, J.; Wu, H. Facile Preparation of Porous Magnetic Polydopamine Microspheres through an Inverse Replication Strategy for Efficient Enzyme Immobilization. J. Mater. Chem. B 2015, 3, 7194–7202. [Google Scholar] [CrossRef]
- Wang, C.; Yan, J.; Cui, X.; Cong, D.; Wang, H. Preparation and Characterization of Magnetic Hollow PMMA Nanospheres via in Situ Emulsion Polymerization. Colloids Surf. A Physicochem. Eng. Asp. 2010, 363, 71–77. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, J.; Caruntu, D.; Yu, M.H.; Chen, J.F.; O’Connor, C.J.; Zhou, W.L. Fabrication of Magnetic Porous Hollow Silica Drug Carriers Using CaCO3 Fe3O4 Composite Nanoparticles and Cationic Surfactant Double Templates. J. Appl. Phys. 2008, 103, 07A320. [Google Scholar] [CrossRef]
- Khabibullin, V.R.; Chetyrkina, M.R.; Obydennyy, S.I.; Maksimov, S.V.; Stepanov, G.V.; Shtykov, S.N. Study on Doxorubicin Loading on Differently Functionalized Iron Oxide Nanoparticles: Implications for Controlled Drug-Delivery Application. Int. J. Mol. Sci. 2023, 24, 4480. [Google Scholar] [CrossRef]
- Sritharan, S.; Sivalingam, N. A Comprehensive Review on Time-Tested Anticancer Drug Doxorubicin. Life Sci. 2021, 278, 119527. [Google Scholar] [CrossRef] [PubMed]
- Christidi, E.; Brunham, L.R. Regulated Cell Death Pathways in Doxorubicin-Induced Cardiotoxicity. Cell Death Dis. 2021, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Lin, P.Y.; Hsieh, S.L.; Kirankumar, R.; Lin, H.Y.; Li, J.H.; Chen, Y.T.; Wu, H.M.; Hsieh, S. Utilizing Edible Agar as a Carrier for Dual Functional Doxorubicin-Fe3O4 Nanotherapy Drugs. Materials 2021, 14, 1824. [Google Scholar] [CrossRef] [PubMed]
- Nieciecka, D.; Celej, J.; Żuk, M.; Majkowska-pilip, A.; Żelechowska-Matysiak, K.; Lis, A.; Osial, M. Hybrid System for Local Drug Delivery and Magnetic Hyperthermia Based on Spions Loaded with Doxorubicin and Epirubicin. Pharmaceutics 2021, 13, 480. [Google Scholar] [CrossRef]
- Norouzi, M.; Yathindranath, V.; Thliveris, J.A.; Kopec, B.M.; Siahaan, T.J.; Miller, D.W. Doxorubicin-Loaded Iron Oxide Nanoparticles for Glioblastoma Therapy: A Combinational Approach for Enhanced Delivery of Nanoparticles. Sci. Rep. 2020, 10, 11292. [Google Scholar] [CrossRef]
- Cai, W.; Guo, M.; Weng, X.; Zhang, W.; Chen, Z. Adsorption of Doxorubicin Hydrochloride on Glutaric Anhydride Functionalized Fe3O4@SiO2 Magnetic Nanoparticles. Mater. Sci. Eng. C 2019, 98, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Khan, M.F.A.; Rahdar, A.; Hussain, S.; Tareen, F.K.; Salim, M.W.; Ajalli, N.; Amirzada, M.I.; Khan, A. Design and Evaluation of PH Sensitive PEG-Protamine Nanocomplex of Doxorubicin for Treatment of Breast Cancer. Polymers 2022, 14, 2403. [Google Scholar] [CrossRef] [PubMed]
- Rahdar, A.; Hajinezhad, M.R.; Hamishekar, H.; Ghamkhari, A.; Kyzas, G.Z. Copolymer/Graphene Oxide Nanocomposites as Potential Anticancer Agents. Polym. Bull. 2021, 78, 4877–4898. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Hoseinzadeh, H.; Labib, P.; Jabbari, P.; Mohebbi, A.; Barzeger, S.; Jafari, H. (Magnetic Laponite/κ-Carrageenan)@chitosan Core–Shell Carrier for pH-Sensitive Release of Doxorubicin. Polym. Bull. 2023. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Ahmadi, M.J.; Dinani, H.S.; Ajalli, N.; Dorkoosh, F. Theranostic Applications of Stimulus-Responsive Systems Based on Fe2O3. Pharm. Nanotechnol. 2022, 10, 90–112. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, E.P.; Lazarin-Bidóia, D.; Bini, R.D.; Nakamura, C.V.; Cótica, L.F.; de Oliveira Silva Lautenschlager, S. Doxorubicin-Loaded Iron Oxide Nanoparticles Induce Oxidative Stress and Cell Cycle Arrest in Breast Cancer Cells. Antioxidants 2023, 12, 237. [Google Scholar] [CrossRef]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic Extracellular Microenvironment and Cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santhamoorthy, M.; Vy Phan, T.T.; Ramkumar, V.; Raorane, C.J.; Thirupathi, K.; Kim, S.C. Thermo-Sensitive Poly (N-Isopropylacrylamide-Co-Polyacrylamide) Hydrogel for PH-Responsive Therapeutic Delivery. Polymers 2022, 14, 4128. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Inoue, S.; Ljubimov, A.V.; Patil, R.; Portilla-Arias, J.; Hu, J.; Konda, B.; Wawrowsky, K.A.; Fujita, M.; Karabalin, N.; et al. Inhibition of Brain Tumor Growth by Intravenous Poly (β-L-Malic Acid) Nanobioconjugate with PH-Dependent Drug Release. Proc. Natl. Acad. Sci. USA 2010, 107, 18143–18148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovrigina, E.; Chubarov, A.; Dmitrienko, E. High Drug Capacity Doxorubicin-Loaded Iron Oxide Nanocomposites for Cancer Therapy. Magnetochemistry 2022, 8, 54. [Google Scholar] [CrossRef]
- Wang, H.T.; Chou, P.C.; Wu, P.H.; Lee, C.M.; Fan, K.H.; Chang, W.J.; Lee, S.Y.; Huang, H.M. Physical and Biological Evaluation of Low-Molecular-Weight Hyaluronic Acid/Fe3O4 Nanoparticle for Targeting MCF7 Breast Cancer Cells. Polymers 2020, 12, 1094. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.; Bin Ahmad, M.; Haron, M.J.; Namvar, F.; Nadi, B.; Ab Rahman, M.Z.; Amin, J. Synthesis, Surface Modification and Characterisation of Biocompatible Magnetic Iron Oxide Nanoparticles for Biomedical Applications. Molecules 2013, 18, 7533–7548. [Google Scholar] [CrossRef] [Green Version]
- Yallapu, M.M.; Foy, S.P.; Jain, T.K.; Labhasetwar, V. PEG-Functionalized Magnetic Nanoparticles for Drug Delivery and Magnetic Resonance Imaging Applications. Pharm. Res. 2010, 27, 2283–2295. [Google Scholar] [CrossRef] [Green Version]
- Shete, P.B.; Patil, R.M.; Tiwale, B.M.; Pawar, S.H. Water Dispersible Oleic Acid-Coated Fe3O4 Nanoparticles for Biomedical Applications. J. Magn. Magn. Mater. 2015, 377, 406–410. [Google Scholar] [CrossRef]
- Niu, Y.Q.; Liu, J.H.; Aymonier, C.; Fermani, S.; Kralj, D.; Falini, G.; Zhou, C.H. Calcium Carbonate: Controlled Synthesis, Surface Functionalization, and Nanostructured Materials. Chem. Soc. Rev. 2022, 51, 7883–7943. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Zhang, T.; Peng, M.; Yi, J.; He, Y.; Fan, H. Design of Magnetic Nanoplatforms for Cancer Theranostics. Biosensors 2022, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Ching, Y.C.; Gunathilake, T.M.S.U.; Chuah, C.H.; Ching, K.Y.; Singh, R.; Liou, N.S. Curcumin/Tween 20-Incorporated Cellulose Nanoparticles with Enhanced Curcumin Solubility for Nano-Drug Delivery: Characterization and in Vitro Evaluation. Cellulose 2019, 26, 5467–5481. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, B.; Xie, S.; Yang, B.; Xu, Q.; Tan, J. Superparamagnetic Iron Oxide Nanoparticles Modified with Tween 80 Pass through the Intact Blood-Brain Barrier in Rats under Magnetic Field. ACS Appl. Mater. Interfaces 2016, 8, 11336–11341. [Google Scholar] [CrossRef]
- Ayub, A.; Wettig, S. An Overview of Nanotechnologies for Drug Delivery to the Brain. Pharmaceutics 2022, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Vergaro, V.; Pisano, I.; Grisorio, R.; Baldassarre, F.; Mallamaci, R.; Santoro, A.; Suranna, G.P.; Papadia, P.; Fanizzi, F.P.; Ciccarella, G. CaCO3 as an Environmentally Friendly Renewable Material for Drug Delivery Systems: Uptake of HSA-CaCO3 Nanocrystals Conjugates in Cancer Cell Lines. Materials 2019, 12, 1481. [Google Scholar] [CrossRef] [Green Version]
- Bondarenko, L.; Terekhova, V.; Kahru, A.; Dzhardimalieva, G.; Kelbysheva, E.; Tropskaya, N.; Kydralieva, K. Sample Preparation Considerations for Surface and Crystalline Properties and Ecotoxicity of Bare and Silica-Coated Magnetite Nanoparticles. RSC Adv. 2021, 11, 32227–32235. [Google Scholar] [CrossRef]
- Ibarra, J.; Melendres, J.; Almada, M.; Burboa, M.G.; Taboada, P.; Juárez, J.; Valdez, M.A. Synthesis and Characterization of Magnetite/PLGA/Chitosan Nanoparticles. Mater. Res. Express 2015, 2, 95010. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The Kinetics and Mechanisms of Amorphous Calcium Carbonate (ACC) Crystallization to Calcite, via Vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Bin Cai, G.; Chen, S.F.; Liu, L.; Jiang, J.; Bin Yao, H.; Xu, A.W.; Yu, S.H. 1,3-Diamino-2-Hydroxypropane-N,N,N′,N′-Tetraacetic Acid Stabilized Amorphous Calcium Carbonate: Nucleation, Transformation and Crystal Growth. CrystEngComm 2010, 12, 234–241. [Google Scholar] [CrossRef]
- Bansal, R.; Singh, R.; Kaur, K. Quantitative Analysis of Doxorubicin Hydrochloride and Arterolane Maleate by Mid IR Spectroscopy Using Transmission and Reflectance Modes. BMC Chem. 2021, 15, 1–11. [Google Scholar] [CrossRef]
- Liang, J.; Yang, X.; Liu, D.; Cong, M.; Song, Y.; Bai, S. Lipid/Hyaluronic Acid–Coated Doxorubicin-Fe3O4 as a Dual-Targeting Nanoparticle for Enhanced Cancer Therapy. AAPS PharmSciTech 2020, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Huang, L.; Xiang, R.; Ou, H.; Li, X.; Chen, A.; Liu, Z. Blood Compatibility Evaluations of CaCO3 Particles. Biomed. Mater. 2021, 16, 055010. [Google Scholar] [CrossRef]
- Nigam, S.; Chandra, S.; Newgreen, D.F.; Bahadur, D.; Chen, Q. Poly(Ethylene Glycol)-Modified PAMAM-Fe3O4- Doxorubicin Triads with the Potential for Improved Therapeutic Efficacy: Generation-Dependent Increased Drug Loading and Retention at Neutral PH and Increased Release at Acidic PH. Langmuir 2014, 30, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Ibiyeye, K.M.; Nordin, N.; Ajat, M.; Zuki, A.B.Z. Ultrastructural Changes and Antitumor Effects of Doxorubicin/Thymoquinone-Loaded CaCO3 Nanoparticles on Breast Cancer Cell Line. Front. Oncol. 2019, 9, 599. [Google Scholar] [CrossRef] [Green Version]
- Vidallon, M.L.P.; Douek, A.M.; Quek, A.; McLiesh, H.; Kaslin, J.; Tabor, R.F.; Bishop, A.I.; Teo, B.M. Gas-Generating, PH-Responsive Calcium Carbonate Hybrid Particles with Biomimetic Coating for Contrast-Enhanced Ultrasound Imaging. Part. Part. Syst. Charact. 2020, 37, 1900471. [Google Scholar] [CrossRef]
- Halder, K.; Sengupta, P.; Chaki, S.; Saha, R.; Dasgupta, S. Understanding Conformational Changes in Human Serum Albumin and Its Interactions with Gold Nanorods: Do Flexible Regions Play a Role in Corona Formation? Langmuir 2022, 39, 1651–1664. [Google Scholar] [CrossRef]
- Corbo, C.; Molinaro, R.; Parodi, A.; Toledano Furman, N.E.; Salvatore, F.; Tasciotti, E. The Impact of Nanoparticle Protein Corona on Cytotoxicity, Immunotoxicity and Target Drug Delivery. Nanomedicine 2016, 11, 81–100. [Google Scholar] [CrossRef] [Green Version]
- Rampado, R.; Crotti, S.; Caliceti, P.; Pucciarelli, S.; Agostini, M. Recent Advances in Understanding the Protein Corona of Nanoparticles and in the Formulation of “Stealthy” Nanomaterials. Front. Bioeng. Biotechnol. 2020, 8, 166. [Google Scholar] [CrossRef]
- Hasan, M.; Zafar, A.; Jabbar, M.; Tariq, T.; Manzoor, Y.; Mahmood, M.; Hassan, S.G.; Shu, X.; Mahmood, N. Trident Nano-Indexing the Proteomics Table: Next Version Clustering of Iron Carbide NPs and Protein Corona. Molecules 2022, 27, 5754. [Google Scholar] [CrossRef] [PubMed]
- Saptarshi, S.R.; Duschl, A.; Lopata, A.L. Interaction of Nanoparticles with Proteins: Relation to Bio-Reactivity of the Nanoparticle. J. Nanobiotechnol. 2013, 11, 12–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, J.; Kuhn, G.; Fichter, M.; Gehring, S.; Landfester, K.; Mailänder, V. Unraveling the in Vivo Protein Corona. Cells 2021, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Chubarov, A.; Spitsyna, A.; Krumkacheva, O.; Mitin, D.; Suvorov, D.; Tormyshev, V.; Fedin, M.; Bowman, M.K.; Bagryanskaya, E. Reversible Dimerization of Human Serum Albumin. Molecules 2021, 26, 108. [Google Scholar] [CrossRef]
- Dobrynin, S.; Kutseikin, S.; Morozov, D.; Krumkacheva, O.; Spitsyna, A.; Gatilov, Y.; Silnikov, V.; Angelovski, G.; Bowman, M.K.; Kirilyuk, I.; et al. Human Serum Albumin Labelled with Sterically-Hindered Nitroxides as Potential MRI Contrast Agents. Molecules 2020, 25, 1709. [Google Scholar] [CrossRef] [Green Version]
- Lisitskiy, V.A.; Khan, H.; Popova, T.V.; Chubarov, A.S.; Zakharova, O.D.; Akulov, A.E.; Shevelev, O.B.; Zavjalov, E.L.; Koptyug, I.V.; Moshkin, M.P.; et al. Multifunctional Human Serum Albumin-Therapeutic Nucleotide Conjugate with Redox and PH-Sensitive Drug Release Mechanism for Cancer Theranostics. Bioorganic Med. Chem. Lett. 2017, 27, 3925–3930. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.V.; Khan, H.; Chubarov, A.S.; Lisitskiy, V.A.; Antonova, N.M.; Akulov, A.E.; Shevelev, O.B.; Zavjalov, E.L.; Silnikov, V.N.; Ahmad, S.; et al. Biotin-Decorated Anti-Cancer Nucleotide Theranostic Conjugate of Human Serum Albumin: Where the Seed Meets the Soil? Bioorganic Med. Chem. Lett. 2018, 28, 260–264. [Google Scholar] [CrossRef]
- Chubarov, A.S.; Zakharova, O.D.; Koval, O.A.; Romaschenko, A.V.; Akulov, A.E.; Zavjalov, E.L.; Razumov, I.A.; Koptyug, I.V.; Knorre, D.G.; Godovikova, T.S. Design of Protein Homocystamides with Enhanced Tumor Uptake Properties for 19F Magnetic Resonance Imaging. Bioorg. Med. Chem. 2015, 23, 6943–6954. [Google Scholar] [CrossRef] [PubMed]
- Chubarov, A.S.; Shakirov, M.M.; Koptyug, I.V.; Sagdeev, R.Z.; Knorre, D.G.; Godovikova, T.S. Synthesis and Characterization of Fluorinated Homocysteine Derivatives as Potential Molecular Probes for 19F Magnetic Resonance Spectroscopy and Imaging. Bioorg. Med. Chem. Lett. 2011, 21, 4050–4053. [Google Scholar] [CrossRef] [PubMed]
- Mariam, J.; Sivakami, S.; Dongre, P.M. Albumin Corona on Nanoparticles–a Strategic Approach in Drug Delivery. Drug Deliv. 2016, 23, 2668–2676. [Google Scholar] [CrossRef] [Green Version]
- Yallapu, M.M.; Chauhan, N.; Othman, S.F.; Khalilzad-Sharghi, V.; Ebeling, M.C.; Khan, S.; Jaggi, M.; Chauhan, S.C. Implications of Protein Corona on Physico-Chemical and Biological Properties of Magnetic Nanoparticles. Biomaterials 2015, 46, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakulkhu, U.; Mahmoudi, M.; Maurizi, L.; Salaklang, J.; Hofmann, H. Protein Corona Composition of Superparamagnetic Iron Oxide Nanoparticles with Various Physico-Chemical Properties and Coatings. Sci. Rep. 2014, 4, 5020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moya, C.; Escudero, R.; Malaspina, D.C.; De La Mata, M.; Hernández-Saz, J.; Faraudo, J.; Roig, A. Insights into Preformed Human Serum Albumin Corona on Iron Oxide Nanoparticles: Structure, Effect of Particle Size, Impact on MRI Efficiency, and Metabolization. ACS Appl. Bio Mater. 2019, 2, 3084–3094. [Google Scholar] [CrossRef] [PubMed]











| Initial Amount Fe3O4, µg/mL | Hydrodynamic Diameter by DLS, nm | Diameter by TEM, nm | PDI | ζ-Potential, mV | TEM Image (Figure 2) | |
|---|---|---|---|---|---|---|
| NP | Agglomerate | |||||
| 4.5 | 130 ± 2 | 13 ± 2 | 107 ± 41 | 0.211 ± 0.006 | −15.0 ± 0.3 | C |
| 1.8 | 118 ± 3 | 16 ± 2 | 68 ± 20 | 0.26 ± 0.01 | −14.9 ± 0.3 | D |
| 0.9 | 130 ± 9 | 18 ± 3 | 61 ± 19 | 0.248 ± 0.004 | −15.5 ± 0.4 | E |
| 0.45 | 121 ± 6 | 22 ± 1 | — | 0.31 ± 0.01 | −15.6 ± 0.5 | F |
| Initial Fe3O4 | 88 ± 5 | 11 ± 4 | 91 ± 23 | 0.221 ± 0.004 | 21.6 ± 0.7 | A |
| Control CaCO3 | 204 ± 8 | 26 ± 3 | — | 0.14 ± 0.02 | −17.3 ± 0.4 | B |
| Fe3O4@CaCO3 Amount, mg/mL | Hydrodynamic Diameter by DLS, nm | Diameter by TEM, nm | PDI | ζ-Potential, mV | Capacity, µg/mg | DOX Loading Efficiency, % | TEM Image Figure 6 | ||
|---|---|---|---|---|---|---|---|---|---|
| NP | Agglomerate | Shell | |||||||
| 3.2 | 133 ± 3 | — | 0.234 ± 0.005 | −14.7 ± 0.7 | 25± 1 | 79.7 ± 0.2 | — | ||
| 1.6 | 130 ± 2 | — | 0.192 ± 0.008 | −13.0 ± 0.2 | 45 ± 2 | 74.7 ± 0.1 | — | ||
| 1.0 | 128 ± 3 | 10 ± 2 | 75 ± 13 | 4.6 ± 0.3 | 0.144 ± 0.006 | −18.8 ± 0.6 | 73.2 ± 0.4 | 73.2 ± 0.4 | A |
| 0.40 | 129 ± 3 | — | 0.130 ± 0.002 | −14.9 ± 0.4 | 160 + 2 | 64.9 ± 0.6 | — | ||
| 0.20 | 135 ± 5 | 11 ± 3 | 125 ± 31 | 18 ± 2 | 0.14 ± 0.01 | −15.0 ± 0.3 | 295 + 2 | 59.4 ± 0.3 | B |
| 0.10 | 111 ± 3 | 9 ± 3 | 149 ± 45 | 46 ± 9 | 0.30 ± 0.01 | −16.5 ± 0.3 | 525 ± 3 | 52.4 ± 0.4 | C |
| 0.050 | 113 ± 5 | 9 ± 5 | 201 ± 35 | 158 ± 46 | 0.28 ± 0.03 | −12.4 ± 0.2 | 1045 ± 10 | 45 ± 2 | D |
| 0.025 | 105 ± 3 | — | 0.29 ± 0.01 | −19.0 ± 0.5 | 1900 ± 27 | 34 ± 4 | — | ||
| Initial Fe3O4@CaCO3 | 121 ± 6 | — | 0.31 ± 0.01 | −15.6 ± 0.5 | — | — | — | ||
| Sample Abbreviation with Capacity | pH 4.0 | pH 5.0 | pH 6.0 | pH 7.4 | ||||
|---|---|---|---|---|---|---|---|---|
| C DOX, % | C DOX, µg/mL | C DOX, % | C DOX, µg/mL | C DOX, % | C DOX, µg/mL | C DOX, % | C DOX, µg/mL | |
| Fe3O4@CaCO3/DOX25 | 98 ± 4 | 2.4 ± 0.1 | 99 ± 1 | 2.48 ± 0.03 | 68 ± 3 | 1.69 ± 0.05 | 60 ± 4 | 1.5 ± 0.1 |
| Fe3O4@CaCO3/DOX45 | 94 ± 6 | 4.2 ± 0.3 | 98 ± 2 | 4.41 ± 0.09 | 49 ± 4 | 2.2 ± 0.2 | 57 ± 4 | 2.6 ± 0.1 |
| Fe3O4@CaCO3/DOX73 | 71 ± 5 | 5.2 ± 0.4 | 65 ± 4 | 4.8 ± 0.3 | 38 ± 1 | 2.8 ± 0.1 | 37 ± 4 | 2.7 ± 0.1 |
| Fe3O4@CaCO3/DOX160 | 65 ± 6 | 10 ± 1 | 59 ± 5 | 9.4 ± 0.8 | 26 ± 2 | 4.2 ± 0.3 | 21 ± 2 | 3.4 ± 0.1 |
| Fe3O4@CaCO3DOX295 | 44 ± 4 | 13 ± 1 | 32 ± 3 | 9.4 ± 0.9 | 17 ± 1 | 5.0 ± 0.3 | 12 ± 2 | 3.5 ± 0.1 |
| Fe3O4@CaCO3/DOX525 | 23 ± 2 | 12 ± 1 | 25 ± 2 | 13 ± 1 | 11.4 ± 0.4 | 6.0 ± 0.2 | 9 ± 3 | 4.7 ± 0.2 |
| Fe3O4@CaCO3/DOX1045 | 23 ± 2 | 24 ± 2 | 25 ± 3 | 26 ± 3 | 11.1 ± 0.8 | 12.1 ± 0.8 | 7 ± 2 | 7.3 ± 0.2 |
| Fe3O4@CaCO3/DOX1900 | 21 ± 2 | 44 ± 4 | 17 ± 1 | 32 ± 2 | 6.8 ± 0.5 | 13 ± 1 | 4 ± 1 | 7.6 ± 0.3 |
| Sample | HeLa | MCF-7 | ||
|---|---|---|---|---|
| IC 50, µM DOX | IC 50, µg/mL Fe3O4@CaCO3/DOX | IC 50, µM DOX | IC 50, µg/mL Fe3O4@CaCO3/DOX | |
| Fe3O4@CaCO3/DOX25 | 3.0 | 49.5 | 5.2 | 60.0 |
| Fe3O4@CaCO3/DOX45 | 1.8 | 22.0 | 4.5 | 41.6 |
| Fe3O4@CaCO3/DOX73 | 1.6 | 13.8 | 2.5 | 16.4 |
| Fe3O4@CaCO3/DOX160 | 1.2 | 7.0 | 2.0 | 9.9 |
| Fe3O4@CaCO3/DOX295 | 2.3 | 5.8 | 2.2 | 7.6 |
| Fe3O4@CaCO3/DOX525 | 2.4 | 2.4 | 3.1 | 4.3 |
| Fe3O4@CaCO3/DOX1045 | 2.5 | 1.9 | 5.2 | 4.1 |
| Fe3O4@CaCO3/DOX1900 | 2.6 | 1.5 | 5.7 | 3.5 |
| DOX | 2.8 | — | 3.1 | — |
| Sample | Initial C HSA mg/mL | Remaining C DOX, % | DOX capacity in Fe3O4@CaCO3 after Incubation, µg/mg | HSA Loading Efficiency, % |
|---|---|---|---|---|
| Fe3O4@CaCO3/DOX160 | 3.2 | 20 ± 2 | 32 ± 3 | 16 ± 1 |
| 32 | 6 ± 1 | 10 ± 2 | 18 ± 2 | |
| Fe3O4@CaCO3/DOX525 | 3.2 | 65 ± 2 | 341 ± 10 | 13 ± 1 |
| 32 | 60 ± 3 | 315 ± 16 | 16 ± 1 | |
| Fe3O4@CaCO3/DOX1900 | 3.2 | 71 ± 2 | 1539 ± 45 | 12.3 ± 0.7 |
| 32 | 64 ± 1 | 1215 ± 19 | 18.5 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popova, V.; Poletaeva, Y.; Chubarov, A.; Dmitrienko, E. pH-Responsible Doxorubicin-Loaded Fe3O4@CaCO3 Nanocomposites for Cancer Treatment. Pharmaceutics 2023, 15, 771. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15030771
Popova V, Poletaeva Y, Chubarov A, Dmitrienko E. pH-Responsible Doxorubicin-Loaded Fe3O4@CaCO3 Nanocomposites for Cancer Treatment. Pharmaceutics. 2023; 15(3):771. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15030771
Chicago/Turabian StylePopova, Victoriya, Yuliya Poletaeva, Alexey Chubarov, and Elena Dmitrienko. 2023. "pH-Responsible Doxorubicin-Loaded Fe3O4@CaCO3 Nanocomposites for Cancer Treatment" Pharmaceutics 15, no. 3: 771. https://0-doi-org.brum.beds.ac.uk/10.3390/pharmaceutics15030771